Reports
LMED28003 Outline of The Innate and Adaptive Immune Systems Report 2 Sample
In early 2030, a new severe acute respiratory syndrome global pandemic was declared. The etiology of this new disease was identified as a novel Henipavirus, a negative-strand RNA virus. Epidemiologists tracing the virus identified Ascot, a suburb of Brisbane to be the "ground zero" for this outbreak. It is believed that the virus originated in flying foxes and was transmitted to horses and then to people. Genetic screening of the virus shows it to be similar to Hendra virus but with several key genetic changes that allows high levels of person-to-person transmission. Transmission of the virus is through respiratory droplets and aerosols. Viral entry into the body has been shown to be through the Human ephrin-A1 receptor which is abundantly found in the epithelium of the lungs.
Task
You are to prepare a 2000 word outline that "Explains the 'innate' and 'adaptive' immune responses to a novel Henipavirus and outline how the non-specific and specific arms of the immune system cooperate to effect an immune response".
- Start with the premise of someone sneezing or coughing on you and work your way through the immune responses, ending with viral clearance and the formation of immunological memory. Hint: the first part of the immune response are your barriers (skin an mucus layers). Most of the virus will get trapped by these before they get into your lungs.
- You can use diagrams and flow charts if they make it easier for you to explain the topic, but remember if you are using a diagram from a journal or textbook etc. you have to reference where it came from.
- References are needed for this assignment. DO NOT reference my lectures or lecture notes. Much of the information in them is from your textbook or other readily available source.
- Remember that this assessment is worth 30% of your final grade. It will require a significant amount of work to complete, so please do not leave this to the end of the term. Work through it as you learn each part of your immune system.
Solution
Introduction
A virus is known as a submicroscopic infectious agent that replicates only inside living cells. This line of viruses can be different and create effects in different forms. Every living organism is affected by this different line of viruses, but innate and adaptive immune responses create protections against these harmful aspects (Okeke, and Uzonna, 2019).However, a new and rare line of viruses is the reason for the different incurable diseases. Any kind of epidemic or pandemic is the reason for these rare lines of the virus that originated from unknown and unusual sources. In this aspect, the novel henipavirus virus has originated in flying foxes. The unusual origin of this particular virus has created unusual actions in living organisms, specifically in human beings. The virus which has been identified is nothing but a negative strand RNA virus. This latest king of the negative RNA virus seems to be the same as the Hendra virus but with different pathogenic modifications. The transmission aspects are also high and this disease is highly transmitted from horses to human beings. In this outline, the innate and adaptive immune response to a novel henipaviral is too detailed for understanding the possible cure for this particular disease.
Content in this respect, there are two kinds of immunity for the assignment helpline which are quite predominant in the case of the human body and these are innate and adaptive immunity includes the internal mechanism which protects the human body from any kind of pathogens the infiltration of the pathogens is inhibited by various which are post by the human body however it must be noted that this kind of variables are not specific to the pathogens these are non-specific as all the kinds of pathogens are prevented from internalization into the body using the innate immune system, for example, the mucus in the nose and the intestine of the human body does not allow the passages to enter inside the system since the pathogens are trapped inside the mucus and cannot enter into the vital systems. This kind of defence system is also regarded as the first line of defence since it is the primary way of preventing the infiltration of pathogens. However, this type of immune system fails to recognise the specific types of pathogens that invade the human body. The skin is another defensive entity of the innate immune system that does not allow pathogens to invade the system. Again, there are some specialised cells like the natural killer cells and the macrophages which aid in protecting the body by eliminating or inactivating the pathogens through specific processes. The adaptive immunity takes some time to function and it operates only after the innate immunity fails to offer protection against the disease. Adaptive immunity is provided mainly by the T cells and the B cells. The B cells produce the antibodies and these entities help in inactivating the pathogens quite effectively. Furthermore, these cells have specific memory which implies that these cells can recognise the pathogens. The pathogenic attributes are identified with the help of adaptive immunity.
The negative strand of the RNA virus is responsible for the disease caused is influenza, mumps, measles, rabies, encephalitis ad others. These diseases are curable and several autoantibodies are capable of curing this type of disease initially in a living body organism. The different aspects found in the negative strand virus in the RNA. there are two types of immunity which are responsible for providing any kind of Defence to the human body. there are various aspects which have been discovered in this respect of innate immunity. The variations of the genetic materials can be examined with the help of the sequencing strategies and hence the discrepancies between the genetic rearrangements of the affected and the unaffected individulas. Additionally, adaptive immunity is frequently employed to detect specific pathogen sequence alterations, similar to immunity patterns. Therefore, the application of these molecular biology approaches aids in the identification of pathogen pathogenic structural variants that are responsible for the emergence of pathogenic disorders. Therefore, cutting edge technology, such as adaptive immunity, allows us to thoroughly envision the components or biological entities that become aberrated and eventually give rise to harmful diseases.
The findings revealed that there was a structural variance in adaptive immunity in the 40 affected people, where a section of the chromosome had translocations caused by the process of incorporation into the pathogenic material.
The outcomes of the targeted long read sequencing assist in determining that chromosome 8 had extensive rearrangements. Furthermore, it was discovered that chromosome 10 and the sex chromosome are directly related, and the differences are inherited in a recessive way. In the instance of the 30 affected patients who were taken into consideration, all of these mutations were previously detected with the use of pathogenic testing, and this was again validated by targeted long-read sequencing. This demonstrates that adaptive immunity is the method of the changes shown in the afflicted people's cases. The adaptive immunity also confirmed that adaptive immunity is also affirmative. The findings are crucial in determining the pathogenic causes of the development of the cases.
Figure 1:Association and the functionalities of the adaptive and the immune responses
(Source- Schon, 2019.)
The pathogens are atfirst dealt by the mast cells. Again the specific response is provided by the natural killer cells that inactivate the pathogens.The pathogens are again engulfed by the macrphage cells.The antigen presenting cells or the APCs like the dendritic cells which aids in the presentation of the antigen to the CD8 and this is a coreceptor of the T cell receptor that this receptors then again activate the CTL.The CTLs provide the immunity by inactivating the pathogens by their cytotoxicity (Mantovani and Garlanda, 2023). Again the antigen prsentation by the dendritic cells can activate the CD4 co receptor.This also helps in activating the major T cells like the Helper T cells and the regulatory Tcells.The Helper Tcells play a major role in combatting the intracellular and the exttracellular pathogens against qall type of infection against the ingresion of the pathogenic entities like the bacteria.Again the autoimmunity is a important factor for the development of the immune responses as this could cause severe damage to the host due to the lack of the recognition of the self antigens.This deleterious affect can aggravate into many specific autroimmune responses that can again take the form of serious disease.The regulatory Tcells helps in regulating and curtailing the self immunity and keeps the self immunity under check.Again the prsentation of the antigens by the dendritic cells are instrumental in the activation of the B cells which in turn lead to the production of the antibodies from the activated B cells.This antibodies are specific in nature and atre produced in response to the specific antigen and helps in responding to the specific antigen.
Furthermore there interlink between the innate and the adaptive iummunity.The antigen presentation cells like the dendritic cells, macrophages, natural killer cell form a bridge between the innate and the adaptive immunity.These cells aid in the activation iof the adaptive immune system entities like the B cells and the Tcells.
Understanding the aetiology in detail is aided by the detailed identification of the chromosomal alterations in the affected patients. Once more, the important findings support the assertion that the adaptive immunity approach used by the immune system is what causes changes to the chromosomal structure (Schon, 2019). To identify pathogenic disorders at an early stage, structural variation identification and its association with the specific pathogenic state are crucial. As a result, these findings would open the way for the creation and enhancement of current approaches for the detection and treatment of infectious disorders.
The analysis of changes in many biological entities, such as proteins, metabolites, transcription factors, etc., makes extensive use of adaptive immunity. These are the variables and entities that have a direct influence on how well an organism functions. For instance, while different proteins are necessary for the body's protein functioning, actin and myosin are in charge of an organism's ability to move. However, there may be some modifications to the pathogenic expressions that alter how these essential proteins operate. Thus, an omics technique like proteomics is crucial for a thorough knowledge of the problem and would aid in precisely identifying how immunity work. The immune system's implications will once more be used to determine the fundamental causes (Khader et al., 2021). Transcriptomics also allows for the estimation of adaptive immunity and aids in the investigation of immunity patterns associated with pathogen functionality and its corresponding impact on protein functions. Consequently, adaptive immunity is frequently employed to identify the relevant variables relating to the various structural and functional defects of the key biological entities that ultimately contribute to the occurrence of the various types of pathogenic illnesses.
According to Sun, Sun, Xiao, and Sun (2019), adaptive immunity has been extensively exploited in the detection of all genomic abnormalities, including pathogenic recombinations or changes in the antibodies. In the establishment of the alterations of antibodies like immunity in this instance, the researcher has carried out extensive tests like adaptive immunity. Once more, adaptive immunity is successful in suppressing harmful manifestations. By using unique immunity probes that can quickly detect the immunity levels in the individual antibodies, adaptive immunity aids in the detection of the specific antigen which can be eliminated by the incorporation of the adaptive and the innate immunity.
Therefore, it can be said that it is crucial to accurately detect adaptive immunity in order to determine whether any crucial protein function has been compromised as a result of the effects of molecular biology approaches like adaptive immunity.
Conclusion
The occurrence of adaptive immunity in the particular pathogenic sequences that result in the silencing of the pathogenic sequencing is established with the aid of adaptive immunity. These organisations aid in identifying the elements needed to close the knowledge gap in defining the numerous pathogenic changes or structural differences that lead to the onset of the diseases. In this context, adaptive immunity, such as the proteomic, must be crucial in identifying the aberrated proteins connected to the onset of any disease, assisting in the early examination of the disease. Again, genomics can be used to illustrate the overall pathogenic modifications or aberrations as it explores the numerous variations in the pathogenic tolerance of individuals.
References
Research
HBD106 Human Biology and Disease Assignment Sample
Individual/Group - Individual
Length - 500 words (+/- 10%)
Learning Outcomes
The Subject Learning Outcomes demonstrated by the successful completion of the task below include:
b) Discuss the role of immunological responses related to inflammation, infection, hypersensitivity, autoimmunity and immunisation.
c) Explain the body’s response to injury and disease at both the cellular and tissue levels.
d) Compare and contrast microorganism types and discuss in the context of immunity, infection control and public health.
e) Identify and describe the pathophysiology, aetiology and clinical manifestations of the common health disorders studied.
f) Explain the underlying pathological and physiological principles as they relate to degeneration and aging.
g) Identify and explore the role social and environmental factors may have in the prevention or pathogenesis of common health disorders.
Task Instructions for Biology Assignment :
To complete this assessment task, your plan must follow these steps:
1) Choose any communicable or non-communicable disease that has been discussed in this course.
2) Research this condition and the bodily systems that it impacts.
3) Outline a plan for your information sheet.
• Your plan for the information sheet should include information about:
(a) Burden of disease e.g., the impact of living with the condition, Disability-Adjusted Life years, Quality-Adjusted Life years, the impact on the medical system and the economy
(b) Clinical manifestations,
(c) Information on investigations and treatments based on the latest scientific evidence.
(i) You can also include any screening tests and links to other helpfulresources.
4) Describe the aetiology and pathophysiological process
5) Identify and utilise medical terminologies/ definitions throughout (remember that your audience is educated medical professionals).
Please also note that
• You are required to present your own original work using multiple academic referencesfrom academic books (at least one), journals (at least 2, published in the last 10 years) and other credible sources. Please see rubric for minimum number of references required for each grade.
• You should present your work as double-spaced text. Dot point entries can be used. Pictures and tables can be useful, however ensure you use correct titles/legends and refer to these in your text.
• Academic references are to be included on a separate page using APA guidelines.
Solution
Introduction
Asthma islong-lasting inflammatory disease, affects the routes of ait in the lungs. Its crucialfeatures include adjustable and repeated indications, reversible flow of airobstacle, and easily activated bronchospasms (Kuruvilla et al., 2019).
Discussion
Burden of disease
The International Study of Asthma and Allergies in Childhood (ISAAC) studied aillustrative sample of 938,687 adolescents between the ages of 13 and 14 in 239 locations in 99 countries between 2020 and 2022 (Dharmage et al., 2019). The most striking finding was the degree to which recent wheeze incidence varied across countries and between areas within those countries. The highest incidence (30%) is often originate in the countries of Australasia, Europe, and parts of Latin America along with North America (Kuruvilla et al., 2019). The bottommostcommonness (7%) was originate in the Indian subcontinent, Eastern Mediterranean, Asia-Pacific, and Northern as well as Eastern Europe (Lambrecht et al., 2019).
.png)
Figure: Asthma (Cleveland Clinic, 2022)
Clinical manifestation of asthma
Some of the clinical signs and symptoms of asthma include recurrent wheezing fits, tightness in the chest, coughing, and shortness of breath (Levy et al., 2006). The symptoms frequently seem harsher at night or immediately after an individual wakes up. They frequently disappear on their own or following an inhaled pain reliever (Boonpiyathad et al., 2019). In spite of the fact that adult asthma deaths are uncommon and mortality rates are falling throughout the majority of European countries, certain really severe episodes can be deadly (Choi et al., 2021).
.png)
Figure: Asthma (Respiratory Health, 2022)
Aetiology and pathophysiology of disease
Although it is common for people to have asthma in their families, neither it is necessary nor sufficient for someone to get asthma (Stern et al., 2020). Anindividual's reaction to ecologicalinductions may also change during the sequence of their lifespan, and the relevant jeopardy factors may change (Menzies-Gow et al., 2021).Asthma attacks the lungs and creates constriction in the airways thus inducing breathlessness among the patient. It has bronchial hyperresponsiveness and variable airway blockage (Hammad & Lambrecht, 2021).
.png)
Figure 1: Pathogenesis of Asthma
(Source: Barcik et al., 2020)
Screening test and treatment
For the purpose of determining how much the bronchial tubes have constricted, a test called spirometry evaluates how rapidly and how greatly air one can exhale after taking a deep breath.Deterrence and longstandingorganization of asthma are critical for stopping asthma occurrences from ever happening (Boonpiyathad et al., 2019). Learning to recognize triggers, taking care to avoid triggers, and monitoring breathing to make sure the medications are managing symptoms are the usual steps in therapy. It could be necessary to use a quick-relief inhaler during an asthma attack (McGregor et al., 2019).
Conclusion
From the overheadargument it can be decided that asthma is one of the greatest prevalent diseases in the western countries. It is triggered by different factors like allergens. It can be chronic as well and might be genetic in most of the cases, and in other cases, they might get worse over timehours, minutes, or even secondsresulting in a more significant obstruction of the airway and an attackor worsening of the asthmathat can only be addressed with more medication.
References
.png)
Case Study
Biology Case study Assignment Sample
Question
Task:
Case Study: Jane
After finishing a practice run, 38-year-old marathon runner Jane showed up at her doctor's office. She works out three days a week, but the previous three runs have left her feeling dizzy and with severe muscle pain. Jane acknowledges to the clinic nurse that she is feeling tired and that her "heart is racing." She was a touch shaky on her feet the night before and stumbled as she climbed the stairs of her house. She admits that she has been consuming fewer calories and less hydration than usual, but continuing to eat properly. In the previous month, she lost 2 kg. She adds that her Garmin watch showed that her resting heart rate used to be 51 bpm.
After her morning run, Jane immediately applied Voltaren Emulgel, a topical NSAID, to her calves and quadriceps in an effort to lessen the pain.
Answering the following question while keeping in mind the case study above
Describe the variations in pressure and volume.
Question 1, will be happening in Jane's chest cavity, in order to achieve exhalation during a lengthy run. Give a justification for these modifications and how they affect airflow.
A. Describe the gas exchange that takes place between the air in Jane's alveoli and the blood in her lungs. Will exercise affect the rate of gas exchange? Justify your response.
Question 2
A. Which ANS reaction would you predict would predominate throughout Jane's runs? Justify your response.
B. Which glucose homeostasis hormone would you anticipate being most active during this ANS response? Why? Explain your response using what you know about glucose homeostasis.
Question 3
A. Describe the function of the kidneys in maintaining fluid balance with reference to the function of antidiuretic hormone in question 3A. (ADH). Is Jane susceptible to failing to sustain homeostatic fluid mechanisms? Whether or not
B. What is a urinalysis and how important is it in this case? Would you anticipate this outcome based on Jane's urinalysis's specific gravity (SG) component and your understanding of typical kidney function? Whether or not
Question 4
A. Take into account Jane's blood pressure reading and examine if the mean arterial pressure is likely to differ from normal. You must indicate a potential change in blood volume and briefly discuss the effects of any BP change on renal function in your response.
B. Which system will be more important in maintaining Jane's blood pressure in this situation, the renin-angiotensin-aldosterone system or natriuretic peptides? Explain your response by describing how the system you choose contributes to blood pressure equilibrium.
Question 5
A. A major hemorrhage occurred during Jane's caesarean delivery, necessitating a blood transfusion. What blood type or types may have been safely given to Jane? Describe what may have happened if Jane had been given A+ blood.
B. Jane had a calcium shortage at the time of her caesarean delivery, it was discovered. What impact would this have had on her blood's ability to clot? Explain your response.
Answer
Introduction
The newest case study in this biology assignment is about Jane, who is brought to the GP clinic at the age of 38 after finishing a training run. She has recently had dizziness, severe muscle pain, and reports of being lethargic, having a racing heart, being unsteady, and other dehydration-related symptoms. She used Voltaren Emulgel to sooth her painful muscles. The case study investigates bodily changes by looking at pertinent homeostasis, the function of the kidney in maintaining fluid balance, and Jane's blood pressure analysis.
Analysis
Long-term adjustments will be made to Jane's chest cavity's pressure and volume to facilitate exhalation. The muscles in the thoracic cavity and the pressure differential between the lungs and the atmosphere both have a role in exhalation. The slightly negative pressure in the chest cavity helps to keep the lungs' airways open. As a result, during exercise, the volume of the chest will significantly expand during inhalation and decrease during exhale. The intercostal muscles are relaxed as a result of the lung recoil, which forces air from the lungs (chest) outside during exhalation. This relaxes the diaphragm, which is located higher in the thoracic cavity and brings the chest wall back to its natural place. The gradient of pressure between the atmosphere and thoracic activity causes the pressure in the thoracic activity to rise in relation to the environment as air rushes out of the lungs (Shao et al., 2014). Since no muscles are contracted to remove air from the lungs, these alterations are thought of as a passive event.
Gas exchange between alveolar air and pulmonary blood takes place over the long term and changes during activity. Inhaled oxygen travels via the lungs to the alveoli. There, the capillaries encircling the alveoli and the layers of cells that line them continue to be in intimate contact with one another. Jane will carry through this procedure quickly while running or exercising to allow for more oxygen and rid the blood of carbon dioxide. The blood in the capillaries' air-blood barrier allows oxygen to travel through quickly. After that, the blood transports the carbon dioxide to the alveoli for exhalation. The oxygenated blood travels from the lungs through the pulmonary veins to the left side of the heart, where it is then pumped to the rest of the body (Qureshi, 2011). After that, blood will be pumped down the pulmonary artery to the lungs, where it will be used to take in oxygen and release carbon dioxide.
The ANS response during Jane's runs comprises the control of the cardiovascular response, which will predominate. The commencement of the somatomotor signal is accompanied by the creation of a cardiorespiratory pattern by the central nervous system (CNS), which is thought of as a central command. This central order causes the heart's parasympathetic activity to decrease, resulting in increased breathing rates, and also resets the arterial baroreflex, resulting in higher pressure. When Jane is jogging, the cardiorespiratory system's main goals are to provide enough oxygen to the bodily tissues and remove waste. Normative blood flow is maintained between all bodily tissues by cardiovascular controls. Running while exercising increases the demand for oxygen to the muscles by 15 to 25 times compared to resting (Liu et al., 2013). The heart cannot function alone because it would be unable to carry out its tasks. Heart rate, blood pressure, and respiratory rates all rise as a result of an increase in the body's need for oxygen. This necessitates significant changes in the blood flow from numerous inert organs towards the skeletal system's active muscles.
The fundamental function of the brain is to control peripheral glucose metabolism via signalling mechanisms and metabolic pathways. Exercises have an effect on several areas of the brain, changing how genes express proteins involved in synaptic plasticity, cellular bioenergetics, neurotrophic factor signalling, cellular stress tolerance, and the removal of harmed organelles and proteins. When the pancreas maintains blood glucose levels that vary within a relatively small range of 4-6 mM throughout the ANS response, the glucagon and insulin hormones are most active (Mitrakou, 2011). The maintenance of glucose homeostasis is accomplished by opposing the balanced activities of glucagon and insulin. The ANS response will cause the glucose homeostasis response to be most active in order to achieve a balance of glucagon and insulin for the maintenance of blood glucose levels.
The kidneys' job is to regulate the urine's concentration so that it reflects the body's demand for water. They do this by creating more diluted urine when the body needs to eliminate surplus water, or they do it by conserving water when the body is dehydrated. Because Jane is dehydrated, her kidneys will retain more water, and the ADH hormone helps the body retain water by improving the kidneys' ability to reabsorb water. By inserting water channels in the kidney tubule membranes, ADH promotes water absorption. The channels subsequently return solute-free water to the blood through the tubular cells, reducing the osmolarity of the plasma and raising the osmolarity of the urine (Cuzzo, & Lappin, 2019). Given that Jane is already dehydrated, she is more likely to fail to maintain a homeostatic fluid mechanism. Osmoreceptors in the hypothalamus monitor the concentration of electrolytes in extracellular fluid to regulate the body's level of hydration. When excessive sweating causes water loss, which causes neuronal signals from osmoreceptors to be transmitted from hypothalamic nuclei, the concentration of these electrolytes in the blood rises. Aldosterone, a steroid hormone generated by the adrenal cortex, is in charge of maintaining the electrolyte concentrations in extracellular fluids. Aldosterone, as opposed to ADH, promotes NA+ reabsorption and K+ secretion from the extracellular fluid in the cells of the renal tubules, assisting in maintaining adequate water balance (Zittema et al., 2012). A drop in blood potassium levels triggers the release of this hormone, halting the loss of Na+ through sweat, saliva, and gastric juice.
A urine test called a urinalysis is used to diagnose and treat a variety of illnesses. The look, concentration, and urine content are all examined. An illness or disease may develop as a result of an abnormal urinalysis. In this instance, the importance of urinalysis is in identifying elevated protein levels or identifying symptoms of kidney disease (Callens & Bartges, 2015). According to Jane's urinalysis results' specific gravity of 1.035 and knowledge of typical kidney functions, an increase in specific gravity in the urine is a sign that the adrenal glands are underproducing hormones, that there is a lot of sodium in the blood, that the person is dehydrated from a loss of body fluids, that the kidney artery is narrowed, or that there is an associated syndrome of inappropriate ADH secretion (Ristic, & Skeldon, 2011). These are the cases that were discovered as a result of Jane's elevated levels of physical activity, together with her ingestion of protein and dehydration.
Given Jane's dehydration, it is more possible that Jane's blood pressure will vary from normal ranges. Due to a reduction in blood volume, dehydration can cause blood pressure to drop. Dehydration causes the blood volume to decrease, which lowers blood pressure since adequate blood volume requires that the blood be able to reach all body tissues. The organs won't obtain the necessary amounts of oxygen and nutrients at such a reduction in pressure levels. The kidneys will lessen the amount of urine produced, which tightens the capillaries in the heart and certain parts of the brain (Daugirdas et al., 2013). It can put a great deal of pressure on the kidney walls since the kidneys won't be able to filter out urine as they normally would under conditions of low blood pressure. Renal disease can result from kidney damage caused by urine retention.
The condition calls for the renin-angiotensin-aldosterone pathway to predominate over natriuretic peptides in maintaining Jane's blood pressure. The RAS controls the fluid balance in the blood as well as blood pressure. Blood potassium levels rise and kidney cells produce the enzyme renin when blood volume or sodium levels in the body fall. Due to the hormone angiotensin I, renin transforms the angiotensinogen generated in Jane's liver. Angiotensin I is converted into angiotensin II by the lung-located enzyme ACE (Provenzano, & Sparks, 2020). In order to restore the potassium, sodium, and fluids and return blood pressure to normal ranges, aldosterone and angiotensin II work to increase blood volume, sodium levels in the blood, and blood pressure.
The blood type that can be safely given to Jane in the event that she haemorrhages during her caesarean delivery and needs a blood transfusion is her blood group. If Jane had received A+ blood, she would have quickly recovered. If Jane had a calcium shortage at the time of her caesarean delivery, it would have prevented her blood from clotting (Fyfe et al., 2012). Calcium ions, the most significant mineral in blood, are required for clotting.
Conclusion
In conclusion, Jane has experienced significant difficulties with her dehydration, which has had a significant negative influence on her kidneys. She needs medical care right away to get her water levels back to normal so that her kidneys and other organs can start working again and her blood pressure will return to normal.
References
.png)
Research
Microbiological Contamination Assignment: Discussion on Remediation
Question
Task: The assignment is designed to evaluate how well you research, and apply contamination control strategies and remediation.
There is no word limit to this assignment.
The assignment is worth 20% of the subject marks.
All assignment work must be the individuals.
Note:
A) The work must be your own and should include a bibliography of source material. Penalties will apply if students submit the same paper.
B) Useful information on preparing assignments is available in the
External Links folder for this subject.
C) All assignments should be uploaded to Turnitin on the submission date specified in your student notes. An assignment cover sheet must be completed and attached to the front of the submitted assignment.
Cover sheets can be found at UTSOnline. You must retain a copy of your submitted work.
Contamination Control Remediation
The attached document is an FDA warning letter related to product contamination that was sent to a pharmaceutical manufacturer.
Your assignment is to examine the warning letter and:
1. Identify the type of contamination detected.
2. Identify the source of contamination
3. Identify the route of transmission
4. Propose practical procedures that would ensure that this type of contamination did not occur again. Categorise these procedures as prevention or detection strategies.
5. Prepare a response to the FDA detailing the corrective action necessary to address all the findings detailed in the Warning Letter.
Answer
Type of Contamination
Microbiological contamination induced by Bacillusthuringiensis or Acinetobacterradioresistens is the sort of contamination that is investigated in the microbiological contamination assignment (Ahmed, 2016).
Source
According to the research done for this biology assignment sample, the creation of foreign proteins and molecules of low molecular weight by the microorganisms Bacillus thuringiensis or Acinetobacter radioresistens is thought to be the source of microbiological contamination. The proteins generated by Bacillus thuringiensis are thought to pose a risk when used as an inexpensive pesticide formulation (purged and at high exposures) or when taken orally in extremely high doses. When administered parenterally in large doses, the decontaminated natural endotoxin that was let loose from these kinds of microorganisms was linked to risk. When an invulnerable traded off individual got infected by the complete, live form of the bacterium, Acinetobacter radioresistens may have been an opportunistic disease (Catellani et al., 2014).
Route of Transmission
The microbiological contamination assignment indicated a pathway of transmission that is anticipated to involve either a small number of bacteria already existing in the system or bacteria that were added during the production process, causing the small inoculum to increase. Non-host cell by-products produced by or as a result of defective high-high limit switches are among the additional channels of transmission (Croughan, Delfosse and Svay, 2014).
What are the practical procedures for the case scenario of microbiological contamination assignment?
Prevention Methods:
1. Risk assessment: This should involve a review of the established facts regarding processing times, in-process endotoxin results, mass drug substance bioburden, the composition of the drug substance as determined by testing, and the system of decontamination processes used during the assembling process.
2. According to the readings used to create this microbiological contamination assignment, crop risk assessments to reflect the greater groups of bacillus thuringiensis to protect drugs from its contamination (Croughan, Delfosse and Svay, 2014).
3. Additional risk assessment via estimation of the quantity and clearance of any potential contamination, toxicological assessment, and data analysis of unfavourable event.
4. Bioreactor disinfection
5. A choice To satisfactorily and successfully remove the filth created by the disinfecting technique under examination, a CIP cycle should be devised and examined.
Detection Techniques
1. Widespread drug substance discharge, stability, and irregular depiction testing
2. Potential impurity level and clearance should be established, toxicological evaluation of identified contaminations should be completed, and adverse event data for the lot should be evaluated.
3. Comparing Soliris drug material batches should undergo explicit investigative testing beyond normal discharge and stability to determine whether any potential pollutions were eliminated throughout the decontamination process.
Investigation of Bioreactor Contamination 4.
5. Additional surface sites should be included for investigative reasons to further develop the ability to identify potential sources of Bacillus thuringiensis and other spore-formers, as specified in the microbiological contamination assignment (Dancer, 2016).
Response Letter
Mr. Leonard Bell,
M.D. Chief Executive
Officer Alexion Pharmaceuticals, Inc.
352 Knotter Drive
Cheshire, CT 06410
March 27, 2013
Amber G. Wardell,
Director of Compliance,
New England District,
Food and Drug Administration,
One Montvale Avenue,
4th Floor, Stoneham,
Massachusetts 02180.
Telephone - 781-***-**84
Subject: Response to FDA Warning Letter- March 22, 2013
Dear Ms. Wardell:
Between July 12, 16-18, 20, 24-26, and August 6, 2012, a CGMP inspection was conducted at Alexion Pharmaceuticals, Inc., which has offices at 352 Knotter Drive in Cheshire, Connecticut, and 100 Technology Way in Smithfield, Rhode Island. Following the conclusion of the inspection on August 6, 2012, an FDA warning letter with Form "483" was delivered along with a number of observations. Alexion Pharmaceuticals, Inc.'s primary goal is to produce products that are both healthy and safe, despite its efforts to maintain order in numerous sectors. The representatives of our company are making an effort to maintain consistency in their work so that they can swiftly examine and modify the tactics and separate the proof and application of office improvements. We endeavoured to take decisive action to eliminate the associated risks at the time when the warning letter was issued, taking the investigators' assertions of perceptions seriously. In addition to trying to take a more thorough look at each component of our organisation, we have tried not to limit ourselves to only the viewpoints expressed in CMS #352798.
The Observations of GMP violations according to FDA's Inspection
If it's not too much of an issue, note that Alexion Pharmaceuticals, Inc. has had systems and tasks which address such observations accordingly for well over a year. These observations were specifically noted in the March 22, 2013 warning letter provided in this microbiological contamination assignment. All of Alexion Pharmaceuticals, Inc.'s cGMP products are subject to certain procedures and tasks, which are as follows:
1. The key deviations and a batch's inability to meet the specifics and the applicable quality requirements were not investigated by your company.
Response
The microbiological contamination assignment's risk assessments make the assumption that the threat to product quality was minimal. The data collected in accordance with SOP QC-0394 and further testing done include:
1. Acinetobacter radioresistens and Bacillus thuringiensis are not thought of as typical human infections (Dancer, 2016).
2. No impact on materials or preparation tools based on the outcomes of the daily practise in-process tests. Bioburden and endotoxin characteristics were all as low as possible:
• The post-filtered bioburden result was 0 CFU/10mL (announced as 1CFU/10 mL) (Shintani, 2015).
• Each of the pre-bioburden testing's results was 0 CFU/10mL (explained as 1CFU/10 mL) (Shintani, 2015).
• The endotoxin findings for all of the pre-tests were 1.25 EU/mL, which, in this case, is the limit of quantification (Shintani, 2015).
• The results for the bioburden and endotoxin were accounted for as 1CFU/10 mL and 0.0625 EU/mL, respectively, which is the highest level of quantification for this case (Shintani, 2015).
• The final mass product substance yields met determinations with aftereffects of 0 CFU/10 mL and 0.1 EU/mg, which serves as the threshold of quantitation in this example (Shintani, 2015).
1. According to the research conducted for the microbiological contamination assignment, the company is focused on finishing the expository tests for the drug material component by April 1, 2013. There were no unexpected results that suggested the presence of bacterial pollutants nearby. The results were expected, and those of the parcels that didn't experience the diagnostic advance had different results.
2. The risk assessments also showed that there was a minimal likelihood of the medicine being co-sanitized with distant proteins or low atomic weight particles that could be given by Bacillus thuringiensis or Acinetobacter radioresistens (Maillard, Sattar and Bradley, 2016).
Further Assessment of Risk
Apart from the elements analysed in SOP QC-0394 and the additional testing discussed above, it was also determined the amount and clearance of probable impurities, completed toxicological evaluations of identified polluting influences, and evaluated data on antagonistic events for the lot.
Worst Case Calculations:
• The calculations for the worst-case scenario, which were covered in the microbiological contamination assignment, were done to determine the quantity of potential impurities that may be produced and the freedom of the potential contaminating influences. in order to initially set up an examination of suspected contaminant expulsion after a bioburden testing. For a 10 mL test, the in-process bioburden testing was too diverse to even consider counting (TNTC) (Maillard, Sattar and Bradley, 2016). The unit activity for the test, as observed from the content produced for the microbiological contamination assignment, took about 15 days. Our working hypothesis is that a small number of microscopic organisms, either already existing in the framework or introduced during the technique, caused this little inoculum to form at that time.
• It was hypothesised that microscopic organisms were introduced into the framework. The bioburden described has increased as a result of the use of Bacillus thuringiensis. The use of Bacillus thuringiensis speaks to the worst-case scenarios in this way. The worst-case situation conceivable was that all of this bulk might include protein contaminations. One pg of protein/mL is concentrated in the drug material lot. As a worst-case scenario estimate, a portion of Soliris limited to 120 mL would contain close to 15 pg of the contaminated protein. While this refers to a fictitious worst-case scenario count, the medication procedure's procedure evacuation information shows that the actual expulsion is a few sets of extent greater.
• The risk assessment stated in the microbiological contamination assignment will be updated to include all discharge, reliability, and additional test results as well as the worst-case scenario count for potential pollution. By March 19th, 2013, the revision will be complete (Rihs, Lee and Stout, 2017).
Toxicology Assessment of Calculated Impurities
• The maximum quantity allowed for a drug substance lot was ="" li="" style="box-sizing: inherit;">
• The concentrations of Acinetobacter radioresistens and Bacillus thuringiensis toxins in the medical section were considerably below any targets determined to be associated with any signs or evidence of harm or other discoveries. Proteins from the Bacillus thuringiensis have been linked to danger when used either as a filter in a readily available bug spray (at high exposures) or when ingested in large doses. When administered parenterally at consistent dosages, the decontaminated endotoxin contained by these kinds of tiny organisms was linked to harmfulness. When a safe bargained human got contaminated by all the living life forms, Acinetobacter radioresistens may have been an opportunistic infection.
• The intentional drug lot has the most recent bioburden of 0 CFU/l0 mL and 0.1 EU/mg and negative endotoxin discoveries evaluated contaminations from bioburden at more than l5 pg of Bacillus thuringiensis per 120 mL portion of medication and all-out conceivable human portion level of a range of 3-8 all out dosages, was surveyed related to the writing search on poisonous impacts of the two microscopic organism (Shintani, 2015).
• The small concentrations of Bacillus thuringiensis proteins linked to any unfavourable effects in either in vivo or in vitro tests are much lower than the large concentrations of bacterial protein segment contamination that have been determined as potentially present in the part. Risk assessments of yields with far greater concentrations of Bacillus thuringiensis assumed that poison crops rather than those that transmitted Bacillus thuringiensis from medication posed no threat to harvest workers or yield buyers. In this way, the risk of clinically unfavourable events connected to the structure of this pharmacological component is regarded as extremely low to negligible.
Adverse Event Evaluation in the context of microbiological contamination assignment
The SOP QC-0394-required hazard analysis for a lot implied that there was little risk to the lot's quality of drugs. The underlying conclusion of safety is supported by other risk assessment methods such as possible impurity amount estimation, toxicological review, and an audit of unfavourable occasion data. The company believes that FDA expects the hazard appraisal to analyse any potential polluting influences produced, such as non-host cell byproducts, in situations where in-process bioburden activity limitations are exceeded, and to establish the procedure polluting influence clearance. Additionally, for the related Soliris medication substance lot, explicit scientific testing beyond routine discharge and dependability needs to be carried out to see whether any prospective contaminating influences made were eliminated during the cleaning process. To ensure that requests for chance appraisals are satisfied, the organisation will assess investigative techniques and methodologies. Future risk assessments that are used to determine the optimum behaviour of lots that undergo an in-process bioburden activity limit trip will include information to satisfy FDA requirements. Alexion will complete SOP QC-0394's update "By March 31, 2013, Bioburden Microbial Risk Management and Assessment (Silbergeld, 2017).
2. Your company has not done enough to stop microbial contamination from happening again during the drug manufacturing process and has not done enough to assess whether the bioreactor contamination episodes are connected.
Response
• In accordance with SOP TMS-0027, "Bioreactor Contamination Investigation," and SOP TMS-0028, "Purification Equipment Investigation," the company has completed an examination of the bioreactor and the occasions of contamination "The methodology expects examinations to be finished by a cross-useful group of topic specialists from Technical Services, Manufacturing, Facilities, Quality Control, and Quality Assurance. Exams conducted in accordance with this SOP are effective inquiries and evaluations of factors to determine the root cause(s) or the most likely root cause (s). The system needs an evaluation of labour, materials, hardware, and environmental factors (Silbergeld, 2017).
•The most likely primary drivers identified with working technique were detected during the deviation examinations in April 2011 and March 2012. In particular, a poor methodology that failed to recognise the possible impact of leftover WFI in a significant amount prior to employing may have contributed to the April 2011 incident. Estimates of counteractive action included adjustments to trustworthiness tests and venting methodologies. To assist with the assessment, the corporation has hired counselling companies. To aid in the completion of research and remediation efforts, generation in the bioreactors has been halted. Initial findings suggest ineffective SIP combined with ineffective routine CIP of non-routine soils. Because of a delay in assembling for safeguard support, non-routine soils were provided, as mentioned in the microbiological contamination assignment. Following the protracted deferral, each bioreactor contamination that occurred in July and August 2012 was initiated. All evidence to date shows that the most recent contaminations' causes are unrelated to the previously established primary driver. Once the examination is complete, the organisation will submit a report to the FDA. By May 1, 2013, the FDA will receive the reports (or an update of the reports that have not yet been completed). There is no correlation between the bioreactor microbial contamination events and the data acquired during all studies. The evaluation will also determine whether there are any shared traits between the early and late instances that call for additional deterrent measures. Additionally, living things isolated from current events like ecological seclusions will be compared to strains that can be found from earlier instances of contamination. The findings will be used to support and rule out putative underlying root causes for ongoing events (Tidswell, Tirumalai and Hussong, 2019).
3. The firm has not appropriately analysed necessitation for an increased frequency of a sporicidal chemical throughout the clean rooms.
Response
• Since January 2011, the corporation has changed its usage of sporicidal operators on two events because of an intensive survey of environmental checking (EM) facts. The frequency of sporicidal cleaning up in the room was increased in response to the outing rate and taking into account the probable impact of generation activities in the context of this microbiological contamination assignment.
• In accordance with the study done for the microbiological contamination assignment, additional surface sites will be included for research to enhance the ability to identify potential sources of Bacillus thuringiensis and other spore-formers. The additional testing locations were chosen to consider several factors, such as proximity to the bioreactor and equipment and potential for heavy staff traffic. Starting on May 9, 2013, additional testing and checking will be done. Depending on the results of the investigation into the bioreactor contamination incidents that occurred in July and August 2012, this may be expanded. The bioreactor decontamination inspection reports or update will include a note with information acquired as a result of the extended examining as one of their main components on April 1, 2013. (Wiencek, 2018).
• Assembling effort will be devoted toward assessing the likelihood of cleaning with sporicidal agents occurring again, and the results will be recorded by way of a risk assessment supporting any following efforts. By August 31st, 2013, the risk assessment will be complete (Wiencek, 2018).
Conclusion
We accept that the activity plans and deadlines described in this response letter discharge our obligation to monitor the 483 and its associated reprimand letter in a comprehensive manner based on the overall analysis performed for the microbiological contamination assignment. All applicable staff members who will be affected by the upgrades have undergone extensive training in conjunction with changes to approaches and tactics. Within fifteen (15) working days of our receiving your notification letter, we will give you a free adjustment of our beneficial exercises.
Additionally, we ask that the FDA publish our response to the Warning Letter on the FDA website. If it's okay with you, consider this letter to be a recommendation for publication on the FDA website.
Sincerely,
/S/ /S/
References
.png)
Assignment
Elisa Test, Its Development, Uses, Procedure and Types Assignment Sample
Question
Task: What is understood by Elisa Test? How did it develop? Procedure to conduct Elisa, its uses and types.
Answer
Introduction
Elisa, an enzyme-linked immunosorbent assay, is regarded as a potent method for isolating and measuring a specific protein from a particular complicated mixture. It is a commonly used technique to determine and find proteins in a specific sample. According to Biology Assignment Help specialists the technique is called an immunoassay because antibodies aid in the detection of the proteins. The Elisa Test is employed as a diagnostic tool in plant pathology and pharmaceuticals. In several sectors, quality control is one of its applications. Indirect, direct, sandwich, and competitive/inhibitory Elisa tests are all variations of the Elisa test. It is regarded as a fundamental, adaptable, quantitative, and sensitive test that aids in determining the concentrations of serum antibody (Vencia, Migone & Vito, 2016). The paper will aid in comprehending the Elisa Test concept, its history, and a discussion of its various forms.
What is Understood by Elisa Test?
The Elisa Test is a device that aids in identifying and quantifying the antibodies that are present in blood. When someone is ill or has a condition, the test is useful for identifying the existence of antibodies in their body. The protein that the body produces in response to dangerous things like antigens is what is known as an antibody. The Elisa Test may occasionally be used as a screening tool before doing any other tests. Engvall and Perlmann first used the phrase in 1971, describing it as a method that helps identify antibodies in a protein sample that has been immobilised in microplate wells (Gandikota, Gandhi & Maisam, 2020). The test aids in measuring glycoproteins and aids in the diagnosis of HIV infection, pregnancy tests, the diagnosis of the chicken pox, zika, and rota viruses, among other things.
Development of Elisa Test
Radioimmunoassy was employed on radioactively labelled antigens and antibodies prior to the creation of the Elisa Test. The presence of any antigen or antibody was employed to detect radioactivity. However, some studies were able to predict some health concerns associated with the use of radioactivity, which prompted researchers to look for alternatives. Two different teams led by Stratis Avrameas and G.B. Pierce created the method known as "enzyme linkage" in 1960. In the same year, Wide and Jerker Porath also published an immunosorbent method. Elisa Test was created as a result of independent studies published by Anton Schuurs and B. van Weemen in the Netherlands and by Peter Perlman and Eva Engvall at the University of Stockholm in Sweden (Gandikota, Gandhi & Maisam, 2020). In the classic Elisa, chromogenic reporters were used together with certain substrates to assist change the colour and signal the presence of a particular antigen or analyte. The new method produced signals using fluorogenic, electrochemiluminescent, and quantitative PCR reporters. When detecting many analytes in a single or cluster of assays and requiring higher sensitivities, the use of advanced reporters is advantageous. The majority of the time, the more recent assays use reporters other than enzymes without altering the basic assay principles, which caused the assays to be classified as Elisas.
Procedure to test Elisa
Testing for Elisa involves no complexities; it is a straightforward process. Before the test is performed, a consent form must be signed, and the doctor will assist in outlining the justification for the test's use. In order to take blood samples, a healthcare professional will scrub the arm with an antiseptic. After that, a band will be placed around the arm to apply pressure to the veins and collect the blood in one location (Hoffstetter, Giffin & Brown, 2018). With the use of a needle that is inserted into the vein, a blood sample will be drawn when the veins inflate with blood. Once the needed volume of blood has been drawn, the blood flow will be stopped by replacing the needle with a tiny bandage. The medical professional will instruct you to keep pressure on the area where the needle was inserted; doing so will help to reduce blood flow. Less discomfort is felt during the sample collection process, however the arm may throb.
A laboratory will then conduct an analysis on the acquired sample. A petri plate that already has the specific antigen of the illness or condition for which the sample was taken will be filled with the blood sample by a medical professional or lab worker.
.png)
Source: (Hoffstetter, Giffin & Brown, 2018)
The sample being placed in the plate for the Elisa test is shown in the image above. Both will unite if the blood already contains antibodies to combat the antigen. In order to check and monitor the interaction between the blood and the antigen, the laboratory worker will add an enzyme to the dish. A change in hue indicates the presence of the disease or condition for which the test was performed. The degree of colour change brought on by the addition of enzyme aids the medical staff in quantifying the level of antibody present.
Uses of Elisa Test and Risks Involved
The test is mostly used to find proteins in the body, though it can also check for antigens. The Elisa Test can assist in identifying hormones, bacteria, viruses, allergens, viral fever, and antibodies that the body produces to fight infections. Additionally, it can aid in locating any agent that tries to infect a person.
Despite the fact that the test is straightforward, the subject occasionally runs the risk of contracting an infection, feeling sleepy, having their blood flow continue, etc. In such circumstances, the doctor must be consulted and kept informed of the situation. The test aids in the identification of Covid 19. If such occurrences arise in the near future, it is also vital to inform the doctor (Kamarehei, Khabiri & Saidijam, 2018).
Result Analysis of Elisa
Depending on the analysis done by the facility conducting the test, the results of the Elisa test may differ. Another element that affects the outcome is the condition or illness. When the report is ready, the doctor will go through the findings and assist in interpreting what they mean. Testing positive occasionally means that the disease or condition doesn't actually exist. False positives and false negatives are possible; the former indicate the existence of a condition when none actually exists, and the latter the non-existence of a condition when it actually does (Kamarehei, Khabiri & Saidijam, 2018). Due to this uncertainty, the elisa test may be repeated on a patient within a few weeks, or the doctor may request that some additional delicate tests be performed in order to confirm the diagnosis.
Types of Elisa Test
Immobilizing the sample antigen in the petri dish is the first step in the Elisa test. Direct absorption on the dish's surface or the assistance of an antibody deposited on the plate can both be used to immobilise the antigen. The test is divided into four categories: competitive, sandwich, indirect, and direct.
.png)
Source: (Lauridsen , Holmetoft & Petersen, 2016)
The illustration above clarifies how various elisa tests operate. The alterations included in the process are used to split the categories. The sandwich elisa test has a higher level of sensitivity and durability, making it a potent elisa assay.
Direct Elisa: Compared to other tests, the procedure of detecting the presence of antibodies is quicker since it involves fewer steps. In this method, the antigen is immediately applied to the microtitre plate wells, and then the enzyme that is designated as the primary antibody that recognises the complimentary antigens is added. The test is less likely to be inaccurate because there are fewer procedures and reagents needed to complete it. Although the method does not call for testing a second antibody, there are some specificity-related drawbacks as well. When compared to other elisa tests, antigen immobilisation has a lower specificity, which results in more background noise (Lauridsen , Holmetoft & Petersen, 2016). It occurs because sample proteins and the target protein on the microtitre plate do not specifically interact. Since all of the target proteins are linked together by enzyme-labeled antibodies, the direct elisa is less versatile. The labor-intensive and time-consuming procedure of labelling primary antibodies can have an impact on the immunoreaction. Since there is no secondary antibody, there is less signal amplification, which lowers assay sensitivity. One may say that this method is employed to examine how the immune system reacts to a particular antigen.
Indirect Elisa Test: A subordinate Elisa test Due to the use of an enzyme-labeled secondary antibody that interacts with the primary antibody, this approach exhibits great sensitivity. Since it uses fewer labelled antibodies than direct elisa, it is seen as being more cost-effective. Because the secondary antibody that has been enzyme-labeled bonds to the other primary antibodies, the indirect elisa is more adaptable. Secondary antibodies with anti-species reactivity are typically polyclonal in origin (Lauridsen , Holmetoft & Petersen, 2016). The cross reactivity between a secondary antibody and a bound antigen, which may produce a greater background noise, is another restriction of the indirect elisa. When the secondary antibody is to be incubated, there is an additional step that must be conducted as part of the test. The procedure takes extra time. The indirect elisa technique aids in calculating the overall amount of concentrated antibody present in a particular sample.
Sandwich Elisa: For this technique, capture and detection antibodies are used in pairs. Either a monoclonal or polyclonal antibody may be used. Every antibody has a high degree of epitope specificity, and it has been discovered that this assay works best with antigens that have two epitopes. The antibody pairs must have matched specificities in order for them to attach to various epitopes and produce reliable results. Elisa is detected using direct and indirect methods as a result of the captured antibody mixing with an antigen. Sandwich ELISA testing is used because antigen quantification occurs in both the upper and lower layers of antibodies (Pereira, Cunha & Fernandes, 2020). Because a sandwich elisa has the tendency to produce accurate but unreliable results, it needs to be verified more frequently. Due to the need for matching pairs of antibodies, the test occasionally takes a long time. Elisa plate must be coated with a captured antibody as the first stage in the sandwich assay process. The addition of a sample antigen to the plate in the second stage is followed by the detection of an antibody. Depending on whether the antibody is enzyme-labeled or enzyme-unlabeled, it will either be a direct sandwich elisa or an indirect sandwich elisa. The secondary enzyme-labeled antibody used in the indirect sandwich elisa is found and introduced to bind the primary unlabeled antibody found. In comparison to direct and indirect elisa techniques, sandwich elisa is a more sensitive method (Pereira, Cunha & Fernandes, 2020). The methodology employs both direct and indirect methods, making it more adaptable when used for detection. The test aids in the analysis of complicated samples that are extremely sensitive and specific because it does not require the pre-purification of antigen. However, there are certain drawbacks to this method that must be taken into account. For example, the elisa kit must be verified in advance for reactivity and detection, which can take time.
Competitive/ Inhibition Elisa: This test is also known as blocking elisa and it utilises a plate/surface assay. Although all other elisa techniques can be adapted to fulfil the standards of competitive elisa, it is known as one of the most difficult assays to do. This technique, which is based on a signal produced by the ensuing interference, aids in estimating the concentration of antibodies or antigens in a specified sample (Sahli, Mouelhi & Tlig, 2018). It demonstrates how a given antigen or antibody competes with a labelled antigen or antibody that has a low concentration. The output signal is inversely correlated to the concentration of antigen in a given sample, with weaker output signalling occurring at greater antigen concentrations. The experiment shows how an antigen coats a microtitre plate. Once the ideal blocking and washing procedure has been accomplished, samples of unknown antigens are added. By including labelled detection antibody and substrates like 3,3',5,5'-Tetramethylbenzidine or TMB, it is dragged. The competitive interaction between the sample and the antigen that binds to the multiwall plates with the primary antibody is one of the crucial processes in this test (Sahli, Mouelhi & Tlig, 2018). When the antigen concentration is high, the output signal will be weak, and when the antigen concentration is low, the output signal will be strong. When an antibody is readily available that is specific to the sample antigen, that is when it should be used. As opposed to the sandwich technique, it aids in the detection of all antigen types, no matter how large or little. Before starting the reaction, the sample must be pre-incubated with another component.
Conclusion
It may be said that the Elisa test aids in the discovery of an antigen or an antibody in a particular sample. It assists in determining whether a person has a condition or not, and if so, whether or not he has an antibody to treat the ailment. There are Elisa test kits on the market that include a plate that has already been coated, a detecting antibody, and other chemicals needed to conduct the test. Sandwich elisa tests are among the several types of Elisa tests, and they are thought to be a suitable technique.
References
.png)
Case Study
Constantina Case Study: Adult Female Marathon Runner Assignment Sample
Question
Task:
Question 1 (8 marks total)
A. Endometrial tissue contains glandular structures relevant to Constantina’s reproductive function. Note the role of this glandular tissue in reproductive function, including any likely changes from normal in the secretion and resultant effects for Constantina.
B. Considering Constantina and her current circumstances describe the role of oestrogen and discuss how the levels of this hormone may vary from normal.
Question 2 (8 marks total)
A. Describe the role of the kidneys in maintaining fluid balance with reference to the role of antidiuretic hormone (ADH). Is Constantina at risk of not maintaining homeostatic fluid mechanisms? Why/why not?
B. What is a urinalysis and what is its significance for this case? With respect to the specific gravity (SG) component of Constantina’s urinalysis result, and using your knowledge of normal kidney function, would you expect this result? Why /why not?
Question 3 (8 marks total)
A. What is gut motility? Is it likely that Constantina’s gut motility has increased or decreased from normal? Discuss EITHER peristalsis OR segmentation in your response.
B. Why is it important for Constantina to maintain adequate protein intake? Discuss its importance in cellular recovery in your response.
Question 4 (8 marks total)
A. Constantina has used Voltaren Emugel (containing a NSAID) to ease her aching muscles. Identify the route of administration and discuss how the drug is likely to be absorbed after administration and its likely bioavailability. Justify your answer by discussing whether the drug would be subjected to hepatic first pass.
B. What is the importance of the half-life of a drug? Assuming 100% absorption and the half-life of an NSAID is 8 hours; calculate the % amount of drug that is likely to be present in the blood after 24hours.
Question 5 (8 marks total)
A. Consider Constantina’s blood pressure result and discuss whether the mean arterial pressure is likely to be varied from normal. In your answer you must include reference to a possible change in blood viscosity and briefly mention the consequences of any change in BP upon kidney function B. Discuss the role of the renin-angiotensin-aldosterone system in the maintenance of blood pressure in Constantina’s circumstance.
Answer
The Constantina biology assignment case study seeks to critically examine the clinical data provided by Constantina, a 38-year-old adult female marathon runner who visited a GP office in post-training run state.
Constantina's reproductive function may be considered in the context of endometrial tissue, which has glandular structures important for the release of glycogen, which enhances blood flow in the spiral arteries and raises progesterone levels (Lessey & Young, 2019). According to study by Brame, Macedo, and Klein (2017), high-intensity exercise in women lowers progesterone levels, which in turn lowers glycogen release from the glandular structure of endometrial tissue and may lead to poor endometrium tissue maintenance and monthly imbalance. Regarding the Constantina case study, Constantina engages in vigorous exercise, which has reduced progesterone production and caused glandular tissue to perform poorly in the release of glycogen, adversely impacting the reproductive system and menstrual cycle.
The release of oestrogen, which is crucial for maintaining reproductive health, raising cholesterol levels, and strengthening bones, is connected to the reproductive process (Vellanki, K., & Kramer, 2019). The secretion of luteinizing hormone and follicle-stimulating hormone is stimulated by an increase in oestrogen during the follicular phase of the menstrual cycle, which also maintains the growth of the endometrium. However, Nagai et al. (2016) suggested that excessive exercise may result in an abnormal variation in oestrogen secretion and a decrease in oestrogen secretion. Thus, it may be claimed that Constantina's three days of intense exercise each week decreased the oestrogen hormone's release from the usual level, which led to amenorrhea and impaired reproductive function.
Focusing on the Constantina case study once again, it is found that Constantina exhibits lethargy and a reduction in fluid consumption, both of which may be connected to renal function. In relation to the antidiuretic hormone, it is essential for maintaining fluid balance by controlling the concentration of urine and its excretion by reabsorption of bodily fluids. The anti-diuretic hormone operates on the late distal tubules of the kidneys and the collecting duct to stimulate re-absorption of water, which helps the body retain water (Cuzzo & Lappin, 2019). Aquaporin-2, which tends to increase water transport over the osmotic gradient and preserve hemostasis, is phosphorylated by it. In the provided case study with Constantina, Constantina is at risk of not maintaining fluid balance since her water consumption has apparently been lower than normal, which increases the likelihood that she may get dehydrated. It is also clear from her physical exam, which is depicted in the Constantina case study, that she is dehydrated, as evidenced by her dry lips, dark circles under her eyes, poor skin turgor, and high urine specific gravity. As a result, the fluid imbalance that results from Constantina's dehydration is not corrected.
A urinalysis was performed on Constantina in order to examine the urine sample, which is important for diagnosis. Urinalysis is the process of examining the colour, consistency, and concentration of urine. It is used to diagnose and treat a variety of illnesses, including kidney problems, UTIs, and diabetes (Free, 2018). The specific gravity (SG) of the urine in the Constantina case study is stated to be 1.035, indicating a value in the upper range. Due to the malfunction of the renal tubules and the production of the ADH hormone, it is thus suggestive of impaired kidney functioning. Due to an increase in urine output and a rise in solute concentration brought on by the inhibition of water reabsorption, there is an excessive loss of water and dehydration (Perrier et al., 2017). Therefore, a high SG is anticipated in the urinalysis because Constantina runs the risk of not maintaining a fluid mechanism.
Another element essential to preserving a person's health is their gastrointestinal motility. Simply put, peristalsis, or the movement of the contents inside the digestive system, is a result of the contractions and relaxations of the muscles of the gastrointestinal (GI) tract, which are referred to as gut motility (Beckett et al., 2017). According to Wood (2019), dehydration causes the gut to absorb a lot of water from food being digested, making it harder to excrete the food and obstructing peristalsis movement, which may be helped by a high water volume. Since dehydration is a problem in Constantina, it may be assumed that peristalsis movement will be less than usual.
Maintaining daily calorie intake, repairing cells and tissues, and promoting muscle and body development are the three major purposes of protein in the diet. Constantina competes in marathons, which are basically endurance sports that may cause discomfort and tissue damage. According to Eddens et al. (2017), eating a diet high in protein is necessary to promote cellular recovery, repair damaged tissue, and preserve the integrity of cells. As a result, she must maintain her protein consumption to satisfy her daily calorie demands. Protein also promotes muscle repair, which assists in cellular healing and facilitates the process of restoring strength (Cintineo et al., 2018).
Constantina was found to have severe muscular discomfort and soreness, according to the Constantina case study. Constantina turned to the NSAID-containing Voltaren Emulgel to soothe her sore muscles. Diclofenac is the NSAID component and the active ingredient in this emulgel, and it works by lowering inflammation and alleviating pain. Topical application through the skin is more likely to be absorbed systemically from the GI tract and first pass via the liver. Diclofenac sodium's relative bioavailability is thus related to the size of the region treated, dependent on the total applied dosage as well as the degree of skin moisture, and was 6% of the systematic exposure, indicating 94% lower than oral diclofenac (Gopalasatheeskumar et al., 2017).
It would be appropriate to discuss the significance of a drug's half-life in this situation. According to the definition, it refers to the length of time needed for a drug's plasma concentration to reach 50% of its whole body concentration (Binder & Skerra, 2017). This drug's half-life, which is important in addition to the two other critical parameters of strength and length, is intended to show if drug buildup may arise as a result of numerous dosage practise. Assuming complete absorption and an NSAID's 8-hour half-life, 50% of the medication will be absorbed in the first 8 hours, and the remaining 50%, or 25%, will be absorbed in the following 8 hours (16th hours). Additionally, half of 25%, or 12.5%, will still be present in the blood after 24 hours.
Constantina's blood pressure was again examined and determined to be 87/58 mm of Hg. Typically, the normal blood pressure ranges from 110/70 mm Hg to 120/80 mm Hg. As a result, it was anomalous in the case study situation for Constantina. Mean arterial blood pressure (MABP), which should typically range from 70 to 110 mm Hg, deviated in this situation and showed a lower result. According to study by Zimmerman et al. (2017), a condition where blood viscosity increases results in an increase in total peripheral resistance (TPR), which obstructs blood flow. The relationship between MABP and cardiac output and TPR shows that raising systolic blood pressure is necessary to maintain blood volume. As a result, low BP in the Constantina case study causes the TPR to drop, which in turn causes the blood viscosity to decrease. It results in a rise in blood flow and a decrease in MABP. According to Larsson et al. (2018), a drop in MABP will also result in a drop in blood volume, which will impede blood flow to the glomerulus. As a result, it affects how well the kidneys reabsorb substances.
For Constantina, a quick decrease in blood pressure triggers the renin-angiotensin-aldosterone pathway, which releases renin from the kidney (RAAS). Through activation of the angiotensinogen, which then transforms into angiotensin II, it causes the synthesis of angiotensin I. Aldosterone hormones are released, and they directly affect the kidney (Ghazi & Drawz, 2017). It works by enhancing salt absorption and releasing it into the bloodstream. Therefore, the conclusions drawn from the examination of the Constantina case study make it clear that in Constantina's circumstance, the production of the hormone aldosterone raises the salt level and blood volume, which ultimately raises the blood pressure.
The medical history, physical examination, and pathological tests, including a urinalysis, performed on Constantina provided crucial information concerning the problem of dehydration and hypotension, it can be inferred from the discussion above based on the case study of Constantina. The execution of these basic pathological tests may help to simplify the treatment plans in a manner that will effectively promote her health and wellness.
Reference
.png)
Dissertation
Do Psychopaths Have Abnormal Brains?
Introduction
According to biology assignment help specialists, personality disorder subcategory is psychopathy. Psychopaths make up around 1% of the population. Philippe Pine, one of the pioneers of modern psychiatry, introduced the idea of psychopathy in the 19th century. He spoke about a group of patients who had mania sans délire (insanity without delirium). They were not intellectually challenged, but their lack of moral and behavioural restraint stemmed from brutality, antisocial behaviour, and alcohol and drug misuse (Kiehl and Lushing, 2014). In 1976, Hervey Cleckley published The Mask of Sanity, a book that had a significant impact. He defined psychopathy as a combination of interpersonal, emotional, and behavioural traits and said that psychopaths typically do not exhibit the symptoms or indicators that most people identify with psychopathy. For instance, not all of them have illusions or hear voices. According to Cleckley, psychopaths may pass as normal while yet concealing a mental disease behind a "mask." He notices that psychopaths seem approachable and simple to converse to. In addition, he says that psychopaths often possess great intelligence and the ability to effortlessly deceive people by their manner of speaking rather than actual words. They are more hazardous than those who have been diagnosed with serious mental diseases since their personality issue is concealed and they might look normal (Moscovici, 2011).
A psychopathy test was created in 1980 by Dr. Robert Hare and made available to the public in 1991. This became the "gold standard" for forensic psychiatrists, researchers, and the legal system in identifying the characteristics and behaviours of psychopaths. This was known as the PCL-R (psychopathy check list- revised), also known as The Hare (Egan, 2016). For the exam, Hare prepared a 20-item checklist. For each item, a score is assigned: 0 for not applying to the individual, 1 for somewhat applying, and 2 for completely applying. Inability to accept responsibility for actions, emotional shallowness, callousness and lack of empathy, a tendency toward boredom, a parasitic lifestyle, impulsivity, irresponsibility, lack of behavioural control, behavioural issues in early life, juvenile delinquency, criminal versatility, and a history of crime are among the traits on the list. A psychopath diagnosis would be possible with a score of 30 or above. The standard score for a "regular" individual is often less than 5, whereas the mean score for inmates is 23. (Egan, 2016)
Psychopathy is seen by many scientists and psychologists as a neurodevelopmental condition. One of them is Kent Kiehl, who finished both his doctoral and master's degrees at Hares' lab. By visiting nearby jails with an fMRI (functional magnetic resonance imaging) equipment and exposing the convicts to both violent and neutral words and pictures, he has examined the brains of over 5000 inmates. According to Kiehl, anatomical and functional anomalies in the brain of psychopaths have an impact on their emotions, impulse control, and cognition. Any anomalies in the amygdala, which is involved in the emotional processes, might be linked to psychopathy. Two investigations using neuroimaging provided proof of this (Blair, 2003). In the first research, conducted in 2000, Tiihonen and coworkers compared the association between psychopathy and amygdala volume in violent offenders. They came to the conclusion that high degrees of psychopathy were correlated with amygdala volume reduction. The same result was reached in the second investigation, which was conducted by Kiehl (2001). Additionally connected to alterations in behaviour and psychopathy is the frontal lobe. Phineas Gage, who had frontal lobe injury after an accident, is an example of a person who changed their behaviour. Even if other aspects of her personality stayed the same, this led to a personality change (speech, movement, intelligence etc). Only after his passing was this reason put forward (Damasio et al., 1994).
Another hypothesis is that a person's early years are the root of their psychopathy. Around the age of four, a person begins to acquire the capacity for both deception and empathy. People with personality disorders, however, do not naturally exhibit this, and it is believed that this results from traumatic events or from how adults treat them, for as via abuse or neglect. In 2013, Craparo, Schimmenti, and Caretti conducted research on the early lives of criminal offenders in Italy. The findings indicated that offenders with high PCL-R checklist scores were more likely to have experienced neglect or abuse as a kid. They came to the conclusion that traumatic experiences in childhood may result in the development of psychopathic tendencies. This was in line with a prior research from 1996 that examined psychopathy and aggression among abused and neglected young people. A matched control group of 489 people and an experimental group of 652 people were compared. PCL-R scores were higher in the experimental group than in the control group. These findings indicated a link between childhood maltreatment and neglect and psychopathy (Weiler and Widom, 1996).
According to Chivers (2014), not all psychopaths will go on to conduct crimes, despite the fact that it is estimated that they make up 1% of the population. Due to their charisma and glibness, some go on to have very successful lives as CEOs and professional sports. Therefore, because it is difficult to foretell whether someone will commit a crime, the justice system cannot penalise someone in advance just because they exhibit psychopathy characteristics and behaviours. It is simpler to estimate a person's chance of reoffending after they have crossed that line and committed a crime.
Theories of Psychopathy
There are two basic ideas that explain psychopathy, according to academics. The first theory is Antonio Damasio's somatic marker hypothesis (1994). He proposed that emotional factors influence how decisions are made. Phineas Gage's accident was the first to show that the frontal lobe is connected to decision-making. Damage to this region, particularly to the prefrontal cortex, therefore decreases one's capacity to accomplish that. It was discovered that those who have prefrontal impairment are unable to convey the right emotions. These psychopathological characteristics gave rise to Damasio's idea. He described emotions as a range of physical changes brought on by the brain's reaction to a stimuli. Eventually, the consequences from a prior scenario are linked to emotions and the matching alteration in the body (somatic marker). Therefore, the body impacts decision-making in favour of a specific behaviour if that circumstance were to recur in the future. For instance, the brain is more likely to repeat a behaviour if it previously produced a good mood or physical signal that left the person feeling joyful. It could also have the reverse effect. For instance, the brain is more likely to oppose a person's behaviour in a circumstance that is similar in the future if a previous experience left them feeling dissatisfied or depressed. (2005) Bechara and Damasio The Iowa gambling task research, which looked at the somatic markers in people with various levels of psychopathy, made a suggestion on the connection between the somatic marker hypothesis and psychopathy. It was shown that psychopaths with high PCL-R scores had the same gambling tendencies as those with frontal brain lesions (van Honk et al., 2002).
The violence inhibition mechanism postulated by Blair is the second hypothesis for the origin of psychopathy. Eibl-Eibesfeldt and Lorenz's study served as the foundation for it. According to their hypothesis, animals have evolved systems that allow them to manage aggression (Weber et al., 2008). An illustration of this is when a combative dog backs off after its adversary bares its throat. The system that prevents violence is assumed to be comparable in humans and is thought to be triggered by distress signals. The amygdala is the key neurological region for this notion (Blair, 2001). Consequently, any irregularities may cause a decrease in the capacity for empathy (Weber et al., 2008). Numerous research support the mechanism that prevents violence. One research examined whether young toddlers with psychopathic traits could recognise emotion in facial expressions. There were 37 kids in the research, aged 9 to 15, who attended a school for kids with behavioural issues. The findings indicated that children with psychopathic tendencies have trouble identifying both depressive and terrified facial expressions (Stevens, Charman and Blair, 2001).
The History of Neuroimaging and Brain Mapping
Lesions in persons from trauma or illnesses have produced the earliest data on brain mapping that is now available. There are three well-known cases: Tan, H.M., and Phineas Gage. Tan, whose loss of language was caused by injury to the left frontal brain, pioneered the knowledge of the localization of language abilities. The most well-known example, however, was H.M., whose bilateral medial temporal lobectomy resulted in a lifelong loss of memory for new knowledge. As Savoy continues, MRI (Magnetic Resonance Imaging) provides improved information on the extent of disorders and injuries, despite patient evidence for brain mapping continuing to be a source of significant information (Savoy 2001).
In order to track and record changes in the brain's blood oxygenation, functional MRI (fMRI) was created in 1990. Patients are exposed to stimuli (words, images, movies, etc.), and any activated brain areas are mapped and compared with brains that are at rest. It has shown to be effective, particularly in psychopathy. In criminal psychopaths, the first fMRI research was released in 2001. (Kiehl and Kofman 2011). This research, conducted in 2001 by Kiehl and colleagues, looked at how criminal psychopaths processed their emotions while doing memory tests. Eight psychopaths, eight non-psychopaths, and eight healthy control volunteers were recruited from a maximum-security jail. The convicts were divided into psychopaths and non-psychopaths using the PCL-R from Hare. The findings indicate that anomalies in the limbic system and parts of the frontal cortex are primarily responsible for criminal psychopathy.
Electroencephalography (EEG), which is non-invasive and widely used in neurological illnesses, is another method of mapping the brain. It may either be used to track the reactions to a stimuli or to detect the ongoing electrical impulses (brain waves). These are known as potentials associated to events. The EEG has great temporal resolution, which is a benefit, but it is insufficient for establishing three-dimensional spatial localization (Savoy, 2001). German psychiatrist Hans Berger is credited with discovering the EEG in 1929, according to Tudor & Tudor (2005). The EEG was initially used on July 6, 1929, during a neurosurgery procedure on a 17-year-old. This resulted in a significant leap in neuroscience.
Electroencephalography
EEG anomalies in psychopaths and non-psychopaths were compared in a research done in 2012 by Calzada-Reyes and colleagues. They employed 58 male violent criminals from a Havana City jail. The PCL-R exam revealed that 31 of the offenders (the experimental group) were psychopaths, whereas the other 28 were not psychopaths (control group). Each offender was given a questionnaire concerning their use of drugs and alcohol, the results of which are presented in the table below:
.png)
Table 1 displays environmental influences on the variables for the experimental and control groups (Calzada-Reyes et al., 2012).
This demonstrates that the experimental group (psychopaths) often consumes more alcohol and psychoactive substances. Furthermore, mistreatment occurred when 55 of the perpetrators were children (30 psychopaths and 25 non-psychopaths). All participants were advised to maintain their calm state throughout the continuous EEG recording in order to reduce movement-related signals.
The findings reveal that while the QEEG revealed greater levels of beta activity in the left parieto-temporal regions of the brain and the bilateral occipital areas in the psychopath group, the visual assessment of the EEG revealed no significant differences between the groups. LORETA was used to verify this. The psychopath group showed less alpha activity than the control group did in the left parieto-central and centro-temporal regions. This research supports the notion that neurodevelopmental problems are a contributing factor in the theory that psychopathy is caused by anomalies in the fronto-temporo-limbic system (Calzada-Reyes et al., 2012).
Functional MRI
Müller and colleagues utilised an fMRI in 2003 to examine how happy and negative pictures affected the activity of the brain in psychopaths. They utilised a control group of six healthy males without a history of neuropsychiatric problems as their subjects. Six male psychopathic patients from a high-security mental health hospital made up the experimental group. In order to evaluate the emotional process, images were employed. Examples of good things included ice cream, joyful couples, puppies, etc. Negative imagery included bones, injured humans, and angry animals/faces, whereas neutral pictures included books, structures, and silverware. To assess the contrast that is dependent on blood oxygen levels, a 1.5 Tesla MRI system with a 25-mT gradient system and a head coil was employed using the fMRI technique (BOLD).
According to the findings, pleasant pictures made psychopaths' bilateral activity of their fusiform cortex, parietal cortex, cerebellar hemispheres, temporal and precentral cortex, as well as their unilateral left-sided activation of their gyrus frontalis inferior, rise. Additionally, the medial frontal gyrus and medial temporal gyrus decreased unilaterally on the right side and bilaterally in the occipital brain. In the picture below, this (increased activation in blue and reduction in red).
Figure 1: A brain scan illustrating the variations in mechanisms behind happy emotion According to Müller et al. (2003), they depict the areas of interest where there is a higher level of activation (blue) compared to the control group (red).
The medial temporal gyrus, occipital, and parietal cortex all showed a bilateral increase in activity. Additionally, it was discovered bilaterally on the left side in the superior temporal gyrus and precentral brain. The anterior cingulate, the amygdala, and the inferior and medial frontal gyrus were all larger on the right side. On the picture below, this is shown in blue. The subgenual cingulate, the medial temporal gyrus, and the gyrus fusiformis all showed unilaterally decreased activity on the right side. Additionally, it was discovered unilaterally on the left side in the dorsal cingulate, the parahippocampal gyrus, and the lobulus paracentralis (Müller et al., 2003). This is shown in red.
.png)
Figure 2: A brain scan demonstrating the ways in which positive and negative emotions are processed differently in psychopaths. When compared to the control group (red), the regions of interest in psychopaths (blue) are more activated (Müller et al., 2003).
According to the study's findings, the stimuli caused both an increase and a decrease in the activity of the brain's emotional-related regions. Comparing these differences to the group of healthy controls revealed that they were considerably different. This suggests that neurodevelopment affects psychopathy in some way.
The Prefrontal Cortex in Psychopathy
The frontal lobe is in charge of cognition, planning, and decision-making.
Many scientists think that psychopathy is influenced by frontal brain injury. Phineas Gage was a well-known example of a person whose personality changed as a result of prefrontal brain injury (as mentioned earlier). He was reliable and trustworthy before the disaster. However, because of the injury to his prefrontal brain (PFC), he developed the psychopathy-related qualities of disrespect, unreliability, and impulsivity. Studies investigating the connection between prefrontal injury (mostly ventromedial and orbitofrontal) and a deficiency in social behaviour and decision-making have been conducted since this instance (Hajak et al., 2008).
Numerous studies have examined the PFC's grey matter organisation. Three different participant groups were used in one research by Yang et al.: 16 failed psychopaths (one or more convictions); 13 successful psychopaths (no convictions); and 23 non-psychopaths as a comparison group. When compared to successful psychopaths and the control group, it was shown that the failed psychopaths had a smaller PFC volume. However, when the PFC volume of the successful psychopaths and the control group were examined, there was no discernible difference. Yang and colleagues also looked at the regional cortical thickness in 32 healthy non-psychopathic people and 27 subjects who were psychopathic. They were matched in terms of age, gender, and drug usage. According to the findings, the PFC thickness was lower in the psychopathic group than in the control group. The right frontal and temporal cortices' grey matter was shown to be thinning. These findings were in line with the theory that psychopathy and grey matter volume are related. Ermer and colleagues conducted one of the biggest investigations in 2011. Voxel-based morphometry and multiple regression analysis were used to look at the association between the degree of psychopathy and the grey matter structure in 254 males from a jail. The outcomes revealed a poor connection between the PCL-R checklist and the volume/concentration of grey matter (Koenigs, 2012).
Laakso and associates looked at the relationship between psychopathy and prefrontal volume in 2002. It was discovered that those who exhibited antisocial behaviour had smaller left orbitofrontal, medial, and dorsolateral cortices. However, neither the level of education nor alcohol usage were accounted for. There were thus no discernible changes after these were controlled. This research contradicted the findings of prefrontal volume in psychopathy (Hajak et al., 2008).
In one research, reversal learning was matched to the dysfunctional ventromedial prefrontal cortex in kids with psychopathic tendencies. Children with oppositional defiant disorders (ODD) or attention-deficit/hyperactivity disorders (ADHD) exhibit more violent and antisocial behaviours. Some of these kids also exhibit psychotic characteristics including a lack of regret or shame. Reversal learning is the process through which a person learns to react to stimuli in order to get a reward. They must learn to react to new stimuli in order to get the reward since once the reaction is known, it is no longer rewarding. It is believed that those who exhibit psychopathic tendencies are unable to achieve this. It is proposed that the ventrolateral PFC reacts to events involving conflict after the dorsal medial PFC is engaged, and that lesions on the orbital and PFC are the source of this impairment. In children and adults who exhibit psychopathic tendencies, damage in the ventromedial PFC impairs reversal learning, according to recent research in neuroimaging and lesion data. The ventromedial PFC or the ventrolateral PFC was shown to be dysfunctional in children who display psychopathic tendencies, according to an analysis of fMRI data. Additionally, they looked at a group of kids with ADHD who lacked any indications of psychopathic qualities as well as a control group of healthy kids who lacked both ADHD and psychopathic features. The findings of this research indicate that children with psychopathic tendencies have anomalies in the ventromedial PFC. There was no indication that the ventrolateral or dorsomedial PFCs were dysfunctional (Finger et al., 2008). All except one of the studies listed point to a critical function for the prefrontal cortex in psychopathy.
The Amygdala in Psychopathy
Understanding the strong aggressiveness is crucial since the amygdala is involved in processing emotions. It is crucial for moral thinking, social connection, and rewarding learning as well (Yang et al., 2009).
The amygdala being damaged is another theory for why psychopathy occurs. Individuals may struggle to recognise distress signs in others or trigger fearful reactions as a consequence of the injury (Pardini et al., 2014).
One of the first research in this field examined the amygdala's localization of deformations in psychopaths. A portion of the 86 subjects, who were all from Los Angeles in California, had significant rates of psychopathy. The PCL-R exam was again used to confirm psychopathy. With a PCL-R score of 23 or above, the findings revealed that 27 of these people were psychopaths. A control group of 32 non-psychopaths with a score of 15 or less was employed. The amygdala was examined using MRI analysis. When psychopathic people were compared to the control group, it was shown that there was a reduction in the bilateral amygdala volume. The findings demonstrated that the basolateral, lateral, cortical, and central nuclei of the amygdala contained the majority of the decreases. According to this research, the amygdala is one of the fundamental characteristics of a psychopath. These findings are also in line with past investigations into amygdala lesions, which demonstrate that injury to the amygdala is a source of emotional deficits, which is a characteristic of psychopathy (Yang et al., 2009). Pardini and colleagues conducted more study on this in 2014. Male participants' reduced amygdala size were investigated to see if they had a history of aggressiveness or psychopathic tendencies as children. They also examined whether an increase in the likelihood of violent behaviour would result from the amygdala's volume being reduced. In a longitudinal research conducted in 1986–1987, 503 guys were first picked from the Pittsburgh Youth Study (PYS). Then, 56 of the 503 men were selected at the age of 26 and had histories of violence of different severity, including no violence, transitory significant violence, and chronic serious violence. Using automated segmentation, the size and level of hostility in the amygdala were studied. The findings revealed no discernible association between amygdala volume and the males categorised according to history of severe violence. However, the amygdala sizes were smaller in individuals who had shown greater violent behaviour and psychopathic tendencies between early adulthood and childhood. This indicated that males were more likely to perform violent acts in the future if their amygdala volumes were lower.
The volume of the amygdala and the characteristics of aggressiveness and psychopathic tendencies from infancy to adulthood were compared for the first time in this research.
A different research examines how psychopaths make moral judgements. It is thought that emotion influences moral judgement and stimulates the amygdala's activity. We investigated the association between the brain activity of psychopaths and moral issues using an fMRI. 17 volunteers from a group of people with varying degrees of psychopathy evaluated 10 circumstances using the following categories:
? moral personal - stirring the emotions (for example, should a baby be smothered to protect themselves and other people from a terrorist).
? moral impersonal - less sentimental (for example, should money found in a lost wallet be kept)
? non-moral (for example, what transport is best to take – bus or train).
On the PCL-R exam, the levels of psychopathy varied from 7.4 to 32, with 32 indicating a high level of psychopathy.
It was discovered that individuals with higher PCL-R exam scores had greater amygdala activity decrease during moral decision-making. The medial prefrontal cortex, posterior cingulate, and angular gyrus all showed decreased activity. This is in line with the theory that in psychopaths, the amygdala's normal function is compromised while making moral choices. It is evident in every characteristic of psychopathy, which strongly implies that amygdala anomalies may be a major factor in psychopathy (Glenn, Raine and Schug, 2009).
Dadds and associates looked at fear recognition in juvenile psychopathy in 2006. Both individuals with amygdala injury and psychopathy have the inability to recognise fear. This supports the hypothesis that anomalies in the amygdala are linked to psychopathy. This research investigated the possibility of treating fear recognition deficiencies in children with psychopathic tendencies. 33 boys between the ages of 8 and 15 participated in the first trial, while 65 boys between the ages of 9 and 17 participated in the second. Participants came from Sydney, Australia, schools. According to this research, failing to see the area around other people's eyes causes them to fail to recognise fear. By focusing on the area around the eyes, you may change this. One of the earliest studies to demonstrate this alteration was this one. It should be mentioned, nevertheless, that Richell and colleagues in 2003 discovered that people with psychopathic tendencies were able to recognise emotions on faces by looking at stimulus faces that included just the information from the eye area.
The Temporal Lobe in Psychopathy
The sensory input, language, and memory are all influenced by the temporal lobe. The hippocampus, which is important in short-term memory, is located in the medial temporal lobe. It's thought that aberrant linguistic abilities may be one factor contributing to psychopathy. According to research, semantic processing activities make psychopaths' impairment to language processes more obvious. According to one view, psychopathic people have trouble understanding abstract language. Since abstract words pertain to things like acts, feelings, and ideas, concrete words are those that can be understood by the five senses (touch, smell, sight, taste, and hearing). Event-related potential (ERP) data revealed that psychopaths were unable to discern between tangible and abstract terms, according to Kiehl et al. (1999). This revealed that the difficulty for psychopathic people to distinguish between the terms was due to the frontal-temporal lobes.
An fMRI was used in one research to look at temporal lobe deviations in criminal psychopaths. Eight male convicts with psychopathic tendencies were utilised, all from a maximum security facility in Canada. The PCL-R test was used to gauge the degree of psychopathy, with the control group failing to exhibit any psychopathic characteristics. Age, educational attainment, parental socioeconomic level, and drug usage in the six months before to the research were all comparable. The study's stimulus words were either concrete or abstract and lacked any evocative overtones. The findings supported the premise that psychopathic people processed abstract words much more slowly than a control group. When processing abstract phrases, the psychopathic experimental group had trouble engaging the right anterior superior temporal gyrus. These results agreed with earlier study findings (Kiehl et al 2004).
In a research conducted in 2008 by Hajak and colleagues, this was further supported. 17 psychopathic subjects and 17 healthy controls were contrasted. Each subject was a guy of a comparable age who had not used drugs during the previous six months before the trial. When compared to the control group, psychopaths have less grey matter in the right and left superior temporal gyrus, according to voxel-based morphometry (VBM). The illustration below illustrates this:
.png)
Figure 3: When compared to a control group, it can be shown that psychopaths have different anatomical characteristics in their grey matter. Yellow represents the grey matter loss. glass brain B: a considerable loss of grey matter in both premotor cortex and temporal lobes. C: Bilaterally significant loss of grey matter in the temporal lobes. D: A notable loss of grey matter in the right middle cingulate gyrus (Hajak et al., 2008).
The temporal cortex was shown to have a crucial role in psychopathy.
Other behavioural investigations have looked at temporal lobe epilepsy patients who exhibit certain psychotic characteristics. The patients' animosity decreased and their social interactions improved after the defective temporal lobe was removed. This supports the hypothesis that anomalies in the medial and anterior temporal lobes play a key role in psychopathy, in line with prior findings (Kiehl et al., 2006). In a 2001 research, the relationship between psychopathy and the temporal lobe's posterior hippocampus is investigated. The study had 18 male volunteers who had all received convictions for serious crimes. They were all alcohol dependant and had at least one personality problem. This research used an MRI, and the findings revealed a poor association between the PCL-R scores and the volumes in the hippocampus. This shows that the hippocampus and psychopathy may be related, and it is also thought that the findings of this research may provide some support for the somatic marker idea (Laakso et al., 2001).
Corpus Callosum in Psychopathy
The axons that make up the corpus callosum link the left and right hemispheres of the brain. It has white matter, which enables communication between the various brain areas (Mooshagian, 2008). It is thought that certain aggressive and antisocial criminals have functional abnormalities in the corpus callosum, notwithstanding the paucity of study on the anatomy of the corpus callosum and its role in psychopathy. Of one research, the white matter and corpus callosum structures in psychopaths and a control group were compared. Additionally, it examines if the aberrations are linked to an emotional deficiency, which is a characteristic of psychopathic behaviour. The study included 15 psychopaths as participants and 25 as controls. They were chosen from five temporary work agencies, enabling a demographic match between the experimental group and the control group. An MRI was used to calculate the corpus callosum's white matter length, thickness, and volume. The findings revealed that the experimental group's white matter volume increased by 22.6% when compared to the control group. Additionally, the experimental group's white matter length was 6.9% longer and 15.3% thinner than the control groups. Furthermore, the aberrant callosal anatomy persisted even after drug misuse was under control. This implies that neurodevelopment may have a role in psychopathy (Raine et al., 2003). Transcranial magnetic stimulation (TMS) has also been utilised in recent research to investigate the relationship between aggressiveness and the corpus callosum. The TMS analyses the signal transmission to determine how well the two hemispheres are connected. Psychopaths were shown to have stronger signals between the right and left sides of the brain than a control group. It shows that psychopathic people with violent inclinations have a diminished reaction in the right cerebral hemisphere (Schutter and Harmon-Jones, 2013). These findings point to a connection between aggressiveness and the corpus callosum, which may be related to psychopathy as aggression is one of its characteristics.
Biochemistry of Psychopathy
Biochemistry may also be a factor in the development of psychopathy. These are the chemical reactions that take place in living things. Monoamine oxidase (MAO) is thought to be connected to psychopathy. Through blood platelets, this substance is extensively dispersed throughout the human body. Several studies have linked reduced platelet MAO to personality problems, however the connection is still ambiguous (Lidberg et al., 1985). Lidberg's research looks at platelet MAO activity in psychopaths. He tested 37 male patients from a Stockholm forensic psychiatric clinic between the ages of 23 and 62 versus a control group. At the same time each day, blood samples were drawn, and the presence of MAO was then examined. The findings revealed that the psychopathic group had a very low level of MAO activity, which was to be anticipated given that psychopathy is associated with personality problems.
The prefrontal cortex's glucose metabolism was the subject of another investigation. Comparisons were made between a control group of individuals with similar age and gender and 22 participants who were charged with murder but pled not guilty due to insanity. The findings demonstrated that the prefrontal cortex's glucose metabolism was considerably lower in the killers. Although further research is needed, there were no discernible alterations in the posterior frontal and temporal lobes, suggesting that it is localised to the prefrontal cortex (Raine et al., 1994).
Discussion
Numerous experts think that abnormalities in the brain are a significant factor in psychopathy. Most researchers have reached the same conclusion after looking at structural and functional problems, mostly in the frontal lobe, amygdala, temporal lobe, and corpus callosum. Future research needs to make numerous advancements in our understanding of the relationship between neurodevelopmental abnormalities and psychopathy.
According to the studies included in this review, the prefrontal-temporo-limbic system has a structural and functional role in the development of psychopathy. Overall, it was shown that psychopaths had less grey matter in the prefrontal and temporal cortex when compared to a control group. Numerous studies have shown that the amygdala may have a role in the development of psychopathy. According to the findings, psychopaths have decreased amygdala basolateral, lateral, cortical, and central nuclei. Other research in this area revealed that the amygdala's dysfunction, which causes issues with emotion processing, may possibly be a factor. This is because one of the characteristics of psychopathy is impaired emotion processing. Although it is thought that the corpus callosum is related to psychopathy, further study is necessary. However, it is important to note that the findings of the study conducted by Raine and colleagues in 2003 imply that there may be some participation, and this might serve as a foundation for further investigation in this area. His research looks at the white matter's structure, and although this calls for additional study, it may be crucial to look at its function and, if any, any associations with psychopathy. Studies on the temporal lobe point to a crucial function for it. When a group of psychopaths were exposed to concrete and abstract phrases in comparison to a control group, it was discovered that there were functional deficiencies. When processing abstract phrases, they had difficulty engaging the right anterior superior temporal gyrus. Additionally, this was in line with a prior research by Kiehl et al.A few research have been done on the biochemistry of psychopathy, including ones on monoamine oxidase. There may be a link between them, according to some. However, this also needs further study, and it hasn't been the subject of many current studies since 1980. The investigations tend to imply that psychopathy is caused by a number of interconnected brain abnormalities rather than by a single neurobiological factor. According to Weber et al. (2008), both psychopathic adults and children with psychopathic features have amygdala abnormalities, although it is thought that only adults have orbitofrontal cortex dysfunction while performing activities. This may be evidence of the emergence of psychopaths, but it might simply be the result of drug misuse, which is sometimes connected to psychopathy. Overall, the evidence points to a link between psychopathy and brain abnormalities.
When examining neurobiological studies of psychopathy, it is crucial to take into account a few methodological issues. The definition of a psychopath is one of the key issues. Most research use Hare's PCL-R exam to assess this. Researchers do utilise a variety of cutoff scores, however. For psychopaths, the conventional cutoff score is beyond 30, whereas for non-psychopaths, it is under 20. As opposed to Kiehl et al. (2004), who used a cut-off score of 28 or higher, Yang et al. (2009) used a cut-off score of 23 or higher. According to Koenigs, this is because it is difficult to locate people with high psychopathy scores exceeding 30 in a forensic context (2012). Another methodological problem is to be able to assemble a control group that matches the experimental group in age, education, and history of drug misuse. It could be beneficial to establish a little lower score as a cut off for everyone to follow in order to prevent any objections. These significant elements may have an impact on the structure and operation of the brain. This proved crucial in the research conducted in 2002 by Laakso and colleagues, as an example. In the first trial, neither alcohol intake nor education level were taken into account. The theory was disproved once these effects were taken into account since there were no significant correlations between antisocial behaviour and prefrontal volume. However, there are several studies that take into account factors like age, education, and past drug misuse, and the findings indicate a connection between brain abnormalities and psychopathy. As a result, even if Laakso and colleagues' theory was disproved, there is still a link between brain abnormalities and psychopathy.
Additionally, in several research it is unknown whether a control group was utilised, and in the majority of the studies, the groups had tiny sample sizes. This makes it possible to compare the findings to those of other research that used a comparable number of people, but it also casts some question on whether they are substantial enough to back up the ideas. However, it is challenging to obtain a big enough cohort for study since it is thought that just 1% of people are psychopaths. As a result, it is known whether the functional abnormalities are caused by psychopathy or are just the result of physiological alterations brought on by incarceration. Many of these research are based on failed psychopaths (those who have been convicted).
Additionally, there is not a lot of study on female psychopathy, and the most of the studies that have been identified have looked at men. Researchers' presumption that psychopathic traits may be transferred to females may be to blame for this. Although it is thought that girls are less likely to develop psychopathy than men are, it is unclear how this is shown behaviorally. Additionally, there haven't been many research on how men and women acquire psychopathy and if there are any notable distinctions (Wynn, Hoiseth and Pettersen, 2012). Future research may be able to look into brain anomalies in female psychopaths, but given how uncommon it is in women, it may be challenging.
Conclusion
In summary, the investigations have shown a link between psychopathy and anomalies in the brain, namely in the frontal and temporal cortex, amygdala, and corpus callosum (the prefrontal-temporo-limbic circuit). These deviations might be functional or structural. There are also some theories that the anomalies are chemical in nature, which have received some research backing. To draw a definitive, general judgement on whether psychopaths have defective brains or not, however, further study is required. It's also important to note that people with other mental illnesses, including schizophrenia, also have impairments in similar brain areas. The hypothesis that psychopaths have aberrant brains is supported by the investigations to far, but further study is required for a definitive response.
Bibliography
Research
Question
Task: Prepare a report on the topic 'Fluid mosaic model of membrane structure'.
Answer
Introduction
A cell membrane with the property of being a two-dimensional liquid with mixed composition is referred to as a fluid mosaic model. The hydrophobic components that are incorporated into the development of the membrane give the cell membrane its fluid quality and cause the lipids and proteins to migrate from one side of the membrane to the other. The membrane has a nature that is more fluid. The fluid mosaic model uses the term "mosaic" to describe anything that was created by combining many elements, which explains why it is appropriate for this model of biology assignment. The fact that the cell membrane is made up of several components justifies the use of the word mosaic in the model's name, the fluid mosaic model. The fluid quality of the cell membrane is caused by the presence of phospholipids, which do not form bonds with one another. The phospholipid molecules have a head that is drawn to water, causing it to point in the direction of the cell membrane's outer surface and giving it a hydrophilic quality. There are two directions that the molecules move in. The tail aids in removing the water that forms a bilayer to create non-polar hydrophobia by wading it away. Fatty acids make up the phospholipid tail, which is continually moving. After the creation of the fluid mosaic model, the development of the bilayer became known. The process is weaker since each person goes through it alone (Catala, 2012). The production of cholesterol is one of the many proteins and chemicals that are entrenched in the bilayer. The plasma membrane has a consistency similar to that of vegetable oil that has been put at room temperature, which allows proteins and several other things to travel around it. Understanding the plasma membrane is accomplished using the fluid mosaic model. The cell membrane is made up of several substances, each serving a specific function. The membrane is stabilized by the cholesterol that becomes embedded in its bilayer, preventing it from hardening at lower body temperatures. The plasma membrane of the cells found in animals is also better understood using the fluid mosaic concept. Glycoproteins and glycolipids are formed from the carbohydrate chains that make up the cell membrane's outer layer. The way that carbs are formed varies from person to person, and how they are formed relies on the type of blood that each individual has (Leabu, 2013).
How did Fluid Mosaic Model Come into Existence?
To explain the composition of the plasma membrane, S.J. Singer and G. L. Nicolson developed the fluid mosaic model. It was believed to be a fundamental component of cell membranes that would aid in future research by explaining the information already available regarding the proteins and lipids found in the membrane (Nicolson, 2016). Even though the concept has changed over time, it is still regarded as a valid theory for explaining the nano-structures of many intracellular and cellular membranes seen in the cells of both plants and animals. The arrangement of the particles in the cell membrane is depicted by the fluid mosaic model. Meanwhile, new information about lipid rafts, proteins, and glycoproteins emerged, aiding in the description of the membrane's structure. Due to the ideas mentioned to define the Fluid Mosaic Model, new information about the cell membrane came to light. The mosaic character is given more weight in recent information (Nicolson, 2016).
What are the different organelles of a cell?
The term "cell organelles" refers to various cellular elements present in both plants and mammals. Organelles are the various structures that make up a cell. The membrane that surrounds the cell's organelles has a specific structure and function (Morange, 2013). All of a cell's parts are contained by the cell membrane, which also shields it from the environment. It also facilitates the entry and exit of ion-regulating particles from the cell. The coordination of these cell organelles is necessary for the cell to function normally. The following list of cell organelles has been discussed:
• Endoplasmic Reticulum:
This is a collection of membrane canals that is hydrated. They are viewed as a means of transportation that aids in moving materials inside the cell. A component of the endomembrane system that extends the nuclear envelope is the endoplasmic reticulum. Rough Endoplasmic Reticulum and Smooth Endoplasmic Reticulum are the two forms of the endoplasmic reticulum. Tubules, vesicles, and cisternae make up the rough endoplasmic reticulum. They aid in the synthesis of proteins and are present throughout the cell. An area of the smooth endoplasmic reticulum serves as storage. It aids in the synthesis of lipids and steroids. It also aids in cell detoxification. The Rough Endoplasmic Reticulum has several ribosomes linked to it, which results in an uneven structure and justifies the name of the organelle. The rough endoplasmic reticulum aids in the synthesis of proteins that leave the cell. These proteins are moved to the lumen inside the endoplasmic reticulum, where they undergo extensive shape modification. The Smooth Endoplasmic Reticulum receives the protein through the lumen and processes it further there. The absence of ribosomes in the Smooth Endoplasmic Reticulum contributes to its smooth structure. Due to a lack of ribosomes, it is unable to produce proteins. Making lipids and enzymes is the Smooth Endoplasmic Reticulum's primary job.
Functions of the Endoplasmic Reticulum:
• Produces and secretes steroid hormones
• Assists in the synthesis of lipids such as cholesterol and phospholipid
• Assists in the metabolism of carbohydrates
• Assists in the release of calcium ions, which is crucial for the nervous and muscular systems
• Ribosomes: This structure is where protein synthesis occurs, producing protein and ensuring the survival of living cells. It is made up of several molecules. All cell types, including prokaryotic and eukaryotic cells, have ribosomes. Ribosomes are required by every single cell for the production of proteins. The tiny and big subunits of the ribosomes are comprised of ribosomal RNA and ribosomal proteins. The messenger RNA (mRNA) and the amino acids that are associated with the transfer RNAs (tRNAs) are transported toward the ribosome during the synthesis of proteins. Proteins are made with the aid of amino acids. The Rough Endoplasmic Reticulum is where the ribosomes are affixed. They are floating in the cytoplasm and are unbound. They have no membranes and are quite tiny in size. Two-thirds of ribonucleic acid and one-third of protein make up this substance. They are also known as prokaryotic 70s or eukaryotic 80s, depending on the type of cell they are found in. S stands for size and density. The skin, hair, eyes, and face all contain ribosomes.
Functions of Ribosomes:
• Assembles amino acids to make proteins, which are thought to be a necessary component for a cell to function. Messenger RNA (mRNA) aids in protein synthesis with the support of the nucleus and cytoplasm.
• The messenger RNA (mRNA) polymers are encircled by ribosome subunits in the cytoplasm.
• The proteins are transported outside of the cell by the newly created ribosomes.
• Golgi Apparatus: Both plant and animal cells have the Golgi complex or the Golgi apparatus in their cytoplasm. It is an organelle that resembles a flat, layered sac that changes proteins and aids in their packaging. It consists of a group of membranes that closely collaborates with the endoplasmic reticulum to change proteins and carbohydrates. It typically has 6 cisternae but can have up to 20 in total. Cells of eukaryotic organisms have the Golgi apparatus. It is surrounded by a membrane, the size of which varies between locations. It actively participates in the production, storage, and delivery of goods made by the endoplasmic reticulum. The Golgi apparatus has a pancake-like form due to the folded membranes there. It serves as the home for numerous vesicles that the Smooth Endoplasmic Reticulum produces. Before being circulated throughout the cell, the Endoplasmic Reticulum's produced proteins are processed by the Golgi apparatus. The protein enters the Golgi apparatus from one side and exits from the opposite side toward the cell's plasma membrane with the assistance of the endoplasmic reticulum. One of the recognized models of the plasma membrane is the fluid mosaic model. A cell's Golgi body might vary depending on what it does.
Source: ( Mandira and Kate, 2017) ( Mandira and Kate, 2017)
The Golgi complex has the following functions:
• Absorbing compounds, assisting in secretion, and forming secretory vesicles
• Assisting in the formation of enzymes
• Assisting in the production of hormones
• Assisting in the storage of proteins
• Assisting in the formation of acrosomes
• Assisting in the formation of intracellular crystals
• Assisting in the formation of milk protein droplets
• Assisting in the formation of plant cell walls
• Assisting in the secretion of glycoprotein
• Lysosomes: Enzyme sacs are what are typically referred to as lysosomes. It aids in the digestion of the various lipids and nucleic acids found in a cell. The lysosomes' internal environment has an acidic character. The circumstances found in the lysosome membrane offer the enzymes a favourable environment in which to work. The cytoplasm is where they are located. The lysosome aids in the breakdown of dying cells, organelles, poisons, and food particles. Lysosomes are frequently referred to as suicide sacks. The lysosome buds from the Golgi complex's membrane sacs. One of the functions of lysosomes is to act as a container for the removal of waste ( Pu, Guardia, Keren-Kaplan and Bonifacino, 2016). Both the prevention and the aetiology of various diseases may be affected by the chemicals that process lysosomes.
Source: (Fields, n.d)
Functions of lysosomes:
• Aid in intracellular digestion
• Remove dead cells
• Aid in metamorphosis
• Aid in protein synthesis
• Aid in fertilization
• Aid in the process of ontogenesis
• Aid in the removal of toxins
• Aid in the digestion of food particles
• Aid in the formation of bone cells
What is Kartagener Syndrome, and How is it Caused?
Source: (Plessis and Wahba, (n.d))
A person who has acute sinusitis, bronchiectasis, and situs inverse is said to have Kartagener syndrome. Kartagener syndrome is caused by the improper acquisition of the motor protein dynein. Chest infections and infertility result from the cilia moving improperly (Xu, Gong and Wen, 2017). Biofilms gathered from the air are incorporated into the mucus, causing bacterial infections and tissue damage. Kartagener syndrome-affected males may generate sterile sperms, and they can only become fathers with the assistance of a physician who can inject the sperm cells into the eggs. When there is a genetic flaw, Kartagener syndrome results. When both parents have the illness and pass the syndrome on to their children, it happens.
What is the role of abnormal dynein in causing the syndrome? How it alters the flagella? How it leads to the building of mucus in the airway?
Genetic disease is the cause of Kartagener syndrome. It results from the production of protein dyneins. People who contract the illness experience chronic sinusitis and mucus buildup in the lungs' airways. The development of germs in the mucus is a possibility. If the condition is inherited, it may develop severe bronchiectasis in children or adults, which can lead to respiratory failure. Cilia and flagella, which are affixed to the surface of eukaryotic cells, are also impacted by the illness. The mucus is moved by the cilia, which also aids in clearing the airways of foreign objects. When the cilia are not functioning properly, it can cause respiratory system problems and make breathing more difficult.
How does Kartagener Syndrome Cause Infertility?
Males with Kartagener syndrome may generate sperm that is sterile. Despite normal sperm production, dynein flagella are absent or have shrunk, which has an impact on sperm quality. The best course of action in these circumstances is to visit a physician who can assist the men in becoming fathers by injecting the eggs with their sperm cells.
The Fluid Mosaic Model, its history, and its etymology have all been covered in the current study. The article also includes information on several cell organelles and a mention of Kartagener syndrome.
Reference List
.png)
Assignment
Analysis of Glucose Molecules Assignment Sample
Question
Task: What is a Glucose molecule? Explain its regulations and functions.
Answer
Greek word glucose, which means sweet, is where the word Glucose first appeared. In 1747, a German scientist by the name of Andreas Marggraf separated Glucose from raisins. Johann Lowitz, a different scientist, found that grapes have a different type of Glucose than normal sugarcane. Jean Dumas coined the name "glucose" later in 1883. (Barclay, Cooper, Ginic-Markovic & Petrovsky, 2010). Understanding glucose molecules, their regulation, and their roles will be made easier by the current work and this biology assignment help.
A monosaccharide, which includes the glucose molecule, is also known as a simple sugar. It is one of the three monosaccharides that our body utilizes. It is regarded as one of the crucial carbohydrates in biology. Adenosine triphosphate synthesis is directly aided by it (ATP). ATP is utilized by the body to create energy. The only single molecule that can be used to create energy is this one. As a result, the body needs the needed amount of glucose molecules.
In prokaryotes and eukaryotes, Glucose is regarded as a significant byproduct of photosynthesis because it starts cellular respiration. The organisms may benefit or suffer harm from Glucose. It is utilized by cells to produce adenosine triphosphate (ATP), which gives the body energy. Since hyperglycemia is cytotoxic, it can cause significant internal inflammation. Hyperglycemia is a disorder that occurs when the body has less Glucose than normal. It can be dangerous and occasionally even fatal (Paine, Pithawalla & Naworal, 2019).
The body may monitor the fluctuating levels of glucose molecules and other systems in a few different ways to prevent potentially dangerous circumstances. Diabetes develops when the body can't control the glucose molecules.
How do Glucose Molecules Regulate?
The food's carbohydrate breaks down into simple sugar while you're consuming it. The digestive system may quickly absorb these simple carbohydrates into the blood, which results in a greater blood glucose level (Barclay, Cooper, Ginic-Markovic & Petrovsky, 2010). The pancreas assists in identifying the rising glucose molecules in this circumstance and secretes insulin in response. Insulin aids in controlling and regulating carbs and monitoring the metabolism of fat.
When insulin is released into the bloodstream, it instructs the fat, muscle, and skeletal cells to absorb the blood's glucose molecules. Insulin release from the pancreas ceases when the blood glucose level falls and reaches a safe level.
A person is never able to express or feel how their blood sugar is doing since they cannot sense hyperglycemia. In some circumstances, a person may experience extreme hunger or thirst, urinate more frequently, or even lose consciousness. For diabetics who continue to take insulin, rapid hyperglycemia could result in a hazardous condition (Paine, Pithawalla & Naworal, 2019).
There are times when going without food for a period can cause the blood's level of Glucose to decline. When such a circumstance occurs, the pancreas responds by releasing glucagon, a separate chemical. Glucagon aids in the liver's conversion of glycogen to Glucose, or Glucose that resembles starch, in the blood. As soon as the blood glucose level returns to a safe level, the release of glucagon ceases.
When it comes to controlling the blood glucose level, insulin and glucagon cooperate. Insulin and glucagon act in opposition to maintaining a normal glucose level. Simple or complex signs of hyperglycemia include feeling poorly or being unconscious, brain damage, or even death (Roder, Wu, Liu and Han, 2016). Harm to the kidneys, the heart, the eye or a nerve, as well as some damage to the hands or feet, are additional complications and symptoms. Once hyperglycemia has occurred, these consequences may take some time to manifest. Understanding the regulation of glucose molecules is made easier by the diagram below:
Structure of Glucose
Glucose (C6H1206) is a compound with 6 carbon atoms and an aldehyde group, commonly known as an aldohexose. The presence of glucose molecules might take the shape of an open chain or a ring. The intramolecular interaction between the C atom of the aldehyde and the C-5 hydroxyl group, which results in an intramolecular hemiacetal, creates the ring. When both are present in water, they remain in balance, but when the pH approaches 7, the cyclic one predominates (Paine, Pithawalla & Naworal, 2019). The ring, which has the appearance of a pyran, has 5 carbon atoms and 1 oxygen atom. Cyclic Glucose is also known as glucopyranose. The carbon atoms in the ring are coupled with a side group of hydroxyl to remove the fifth atom, which links back to a carbon atom put outside the ring in the sixth position to produce a group called CH2OH. The illustration below explains the cyclic and acyclic structures of Glucose:
Source: (Barclay, Cooper, Ginic-Markovic and Petrovsky, 2010)
Production of Glucose
Both commercial and natural production of Glucose is possible. The natural process could appear as a byproduct of photosynthesis, which occurs in some prokaryotes and plants. Glycogenolysis is the process by which glycogen breaks down to produce Glucose in both animals and fungi (Barclay, Cooper, Ginic-Markovic and Petrovsky, 2010). In plants, the breakdown manifests as starch. In the liver and kidneys of animals, Glucose is produced.
The commercial procedure can involve the enzymatic hydrolysis of starch to produce Glucose. Certain crops, including maize, potatoes, rice, etc., can be excellent sources of starch. For example, cornflour is frequently utilized in the USA to create glucose molecules from these crops. The enzymatic process occurs in two steps. The enzymes begin to hydrolyze the starch into tiny carbohydrates, which contain glucose molecules in units of five to ten, in one or two hours at 100. C. The starch mixture may occasionally be heated during the process to 130° C or higher (Zhang & Bar-Peled, 2019). The water is heated to assist in dissolving the starch, but heating also deactivates the enzymes, necessitating the addition of additional fresh enzymes after each heating.
The second stage, known as saccharification, uses the glucoamylase enzyme, which is derived from the fungus Aspergillus niger, to completely hydrolyze the partially digested Glucose into glucose molecules. Ph4.0-4.5, at 60°C, and a concentration of carbohydrates weighing 30–35% are necessary for the reaction. If this situation persists for fourteen days, the starch will convert to Glucose with a 96% yield (Rensburg & Ende, 2018). This method can also convert more glucose molecules, but it will use a more diluted solution, which might not be practical. Through the use of filters, the glucose solution produced by this procedure is cleaned before being stored in an evaporator. After multiple crystallizations, a solid form of Glucose is produced.
Functions of Glucose
The metabolism and biosphere both benefit from the widespread usage of Glucose. Compared to some other hexose sugars, Glucose has a weaker ability to react with some proteins that include an amino group. The process is known as glycation results in the destruction or reduction of the function of several enzymes (Dienel, 2018). Proteins produced through glycation are likely to be the source of many acute diabetes-related problems, including blindness and renal failure, but glucose protein added through enzyme regulation may serve a crucial purpose.
Source of Energy
Whether they are bacteria or people, glucose molecules are a fantastic source of energy for practically all living things. Some cells in the body completely rely on
Glucose to produce energy. Both aerobic and anaerobic respiration can utilize the Glucose. A significant portion of the energy used by humans during aerobic respiration comes from carbohydrates, which offer food energy of at least four kilocalories per gramme. Glycolysis converts Glucose into CO2 and water when it comes into touch with the citric acid cycle (Dienel, 2018). Adenosine triphosphate, a type of energy, is produced (ATP). The blood glucose level is controlled by insulin with the aid of additional processes.
Glucose Contained in the Glycolysis
To generate energy for either aerobic or anaerobic respiration, cells use Glucose. The process begins in the early stages of glycolysis, and the first step in it is the phosphorylation of Glucose with the aid of the hexokinase enzyme, which will subsequently break down and release energy. In order to prevent diffusion outside the cell, Glucose is immediately phosphorylated with the aid of the hexokinase enzyme.
As a helper
Glucose serves as a cofactor in the synthesis of proteins and the metabolism of lipids. Some plants and animals can produce more vitamin C thanks to it. The process of glycolysis aids in modifying Glucose for subsequent use. Additionally, glucose molecules aid in the creation of several compounds, including starch, cellulose, glycogen, etc. One of the types of Glucose is lactose, which is found in milk (Dienel, 2018).
As a Source of Absorption
Glucose can be found in dietary carbohydrates as building blocks, in starch, in glycogen, or in conjunction with another monosaccharide as a source of absorption. Additionally, Glucose directly fuels erythrocytes and brain cells (Rensburg & Ende, 2018). Some of them end up in the muscles and liver, where they are stored as glycogen. Additionally, it enters the fat cells, where it is stored as fat. When the body needs energy, it can be drawn from glycogen, which is then converted back into Glucose.
Quick facts about Glucose
From the French word glucose, which means sweet, came to the name glucose. The prefix ose in the word "glucose molecule" designates a carbohydrate.
It is classified as a hexose since it has six carbon atoms. It comes in both linear and cyclic forms.
It is necessary for red blood cells, muscle cells, and the energy delivery of the human brain. Additionally, it can dissolve in water.
The abundant monosaccharide found all around us serves as an energy source for various earthly creatures. It is present in plants in the form of sugar, which is created during photosynthesis.
Additionally, Glucose can create isomers that are similar chemically but have distinct conformations. While L-glucose can be handled synthetically, D-glucose is processed naturally.
The glucose molecule has the chemical formula C6H12O6, which can alternatively be written as CH2O in its simplest form.
References
Barclay, T.G., Cooper, P.D., Ginic-Markovic , M & Petrovsky, N. (2010) Inulin - A versatile polysaccharide with multiple pharmaceutical and food chemical uses. Journal of Excipients and Food Chemicals, 1(3).
Dienel, G.A. (2018) Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev, 99(1).
Paine, J.B., Pithawalla, Y.B & Naworal, J.D. (2019) Carbohydrate pyrolysis mechanisms from isotopic labeling. Part 5. The pyrolysis of D-glucose: The origin of the light gases from the D-glucose molecule. Journal of Analytical and Applied Pyrolysis, 138.
Rensburg, H.C.J & Ende, W.V. (2018) UDP-Glucose: A Potential Signaling Molecule in Plants? Front. Plant Sci.
Roder, P.V., Wu, B., Liu, Y & Han, W. (2016) Pancreatic regulation of glucose molecule homeostasis. Experimental & Molecular Medicine, 48, e219.
Zhang, J & Bar-Peled, L. (2019) How Sweet It Is: Small-Molecule Inhibitors of mTORC1 Glucose Sensing. Cell Chemical Biology, 26(9).
Thesis Writing
Utilizing Fluorescence Microscopy To Characterize The Subcellular Distribution Of The Novel Protein Acheron Assignment Sample
SOLUTION
CHAPTER I - INTRODUCTION
Programmed Cell Death:
The genetic machinery needed to carry out programmed cell death, or cell suicide, is present in every cell (PCD). A variety of crucial functions are served throughout development and homeostasis by the successful beginning of PCD. The aetiology of human diseases including cancer, autoimmune disorders, and neurodegeneration, however, is driven by PCD dysregulation.
In 1842, Karl Vogt made the first-ever observation of cell death. Vogt identified cell death in the notochord and surrounding cartilage in metamorphic toads in his experiments (Vogt, 1842). Beginning with Dreisch's 1895 study of urchin embryo development and Spemann and Mangold's description of "organisers" in the African clawed toad Xenopus laevis, this ushered in a golden era of scientific discovery.
Vogt's discoveries weren't put in the "spotlight" however until almost a century later. In his article "Cell Death in Normal Vertebrate Ontogeny" published in 1951, Alfred Glucksman brought cell death back into the spotlight of developmental study. Glucksman describes his findings of the loss of the notochord core throughout development in this review. Richard Lockshin and Carroll Williams were two among the researchers that were motivated by these discoveries to continue their PCD study.
In the Journal of Insect Physiology between 1964 and 1965, Lockshin and Williams published a collection of five essays titled "Programmed Cell Death." The American silk moth Antheraea polyphemus undergoes eclosion at the conclusion of metamorphosis, and these studies investigated and addressed the neural, cellular, and hormonal processes influencing intersegmental muscle (ISM) cell death. These papers of biology assignment not only represent the first instances of the now-accepted phrase "Programmed Cell Death," but they also sparked a fresh surge of investigation into a hitherto understudied area. Sydney Brenner, Robert H. Horvitz, and John E. Sulston shared the 2002 Nobel Prize in Physiology or Medicine for their studies on the genetic control of organogenesis and programmed cell death in the Caenorhabditis elegans worm. These findings provide light on the crucial functions PCD performs in development and homeostasis. Over 70% of human illness is thought to be caused by deficiencies in PCD control (Reed, 2002). Numerous neurological conditions, including Parkinson's disease, in which striatal dopaminergic neuronal groups die, are caused by inappropriate activation of PCD (Hartman et al., 2000). Conversely, the root cause of many autoimmune disorders and the majority of malignancies is the inability to activate PCD to eliminate dangerous mitotically component cells (Renehan et al., 2001).
Apoptosis is the most well-understood method of cell death. Usually, a family of cysteine proteases known as caspases mediates this process (Miura et al., 1993). Numerous internal and external factors, including as pH changes, hormone exposure or loss, DNA damage, and loss of interaction with other cells, may cause apoptosis to occur (Norburry et al., 2001). Simply put, exposure to these death-inducing stimuli triggers a cascade effect in which effector caspase zymogens are activated by initiator caspases by interchain breakage. The executioner pro-caspases are then activated and cleaved as a result. Executor caspases then break essential proteins in the cell, causing apoptosis and cell death (Elmore, 2007). A closer look shows two primary mechanisms for initiating apoptosis in response to factors that cause death.
The first is the extrinsic route, which is started when members of the tumour necrosis factor (TNF) family of death receptors bind pro-death signalling peptides at the cell membrane (Micheau et al., 2003). Examples of this include ligands that bind to the Fas, DR4/5, and TNFR1 receptors, respectively, such as Fas-ligand (FasL), TNF related apoptosis inducing ligand (TRAIL), and Tumor Necrosis Factor Alpha (TNF) (Arai et al., 2014; Wiley et al., 1995; Cabal-Hierro et al., 2012). The recruitment of death domain/adaptors like Fas-associated protein with death domain (FADD) or Tumor necrosis factor receptor type 1-associated death domain protein is caused by the interaction of pro-death signalling ligands with TNF family pro-death receptors (TRADD). The death-inducing signalling complex (DISC) is created when these molecules are complexed (Scaffidi et al., 1999). Procaspase-8 may be recruited and activated by DISCs via death effector domains (Irmler et al., 1997).
Procaspase-8 is an initiator caspase that has the ability to cleave and activate executioner pro-caspases like caspase-3, -6, and -7, hence starting the PCD execution phase (Elmore, 2007). After then, apoptosis-related morphological and biochemical changes are brought on by activated executioner caspases (Slee et al., 2001). This includes the cleavage of crucial substrates including PARP (poly (ADP-ribose) polymerase) (caspase-3/7) and lamin A/C (caspase-6) as well as DNA fragmentation, nuclear collapse, and cleavage of chromatin (Wajant et al., 2002; Green and Llambi, 2015).
Initiating apoptosis via the intrinsic (mitochondrial) route is the second important process. Many of the same external and internal cues mentioned above may start this route, which is the main mechanism that initiates apoptosis in vertebrates (Bratton et al., 2001, Norburry et al., 2001). Members of the Bcl-2 (B-Cell Lymphoma 2) family of mitochondrial membrane proteins, as well as mitochondrial stress (oxidative stress)/mitochondrial outer membrane permeabilization (MOMP), have a significant impact on PCD in this pathway (Tait and Green, 2010). Three subfamilies may be created from bcl-2 proteins. Pro-apoptotic effector proteins from the first subclass include Bcl-2 associated X protein (Bax) and Bcl-2 homologous antagonist killer (Bak). For MOMP to occur, these proteins are both essential and sufficient (Moldoveanu et al., 2020). Anti-apoptotic regulatory proteins like Bcl-2, B-cell lymphoma-extra-large (Bcl-xL), or Mcl1 are part of the second subfamily (Tsujimoto, 1998). These proteins stop MOMP, which thereby stops cell death. The BH3-only proteins make up the third and last subfamily. These proteins include, but are not limited to, p53 upregulated modulator of apoptosis (PUMA), Bcl-2 interacting protein 3, Bcl-2-like protein 11, Bcl-2 associated agonist of cell death (Bad), and BH3 interacting-domain death agonist (Bid) (Hutt, 2015). By either inactivating antiapoptotic Bcl-2 proteins or activating proapoptotic Bcl-2 proteins, BH3-only proteins promote intrinsic pathway (Delbridge et al., 2016).
MOMP is produced directly by proapoptotic Bcl-2 proteins in the intrinsic cell death pathway (Eskes et al., 2000). Proteins like Bak or Bax oligomerize in the mitochondrial outer membrane after activation, creating holes in the outer membrane (Peng et al., 2009). This triggers MOMP, which releases proteins found in the mitochondrial intermembrane space such as cytochrome c, Smac/Diablo, and Omi (Goldstein et al., 2000; Munoz- Pinedo et al., 2006). Cytochrome C interacts to Apoptotic Protease-Activating Factor 1 (APAF1) after it has entered the cytoplasm (Zou et al., 1997). According to Kim et al. (2005), cytochrome c binding to APAF1 causes the cofactor's dATP to be hydrolyzed to dADP, which then enables the oligomerization of many APAF-1-dATP-cytochrome-c subunits to create an active apoptosome (Yu et al., 2005). Similar to the extrinsic cell death route, the intrinsic cell death mechanism uses caspase enzymes after the production of apoptosomes.
At the core of the apoptosome, Caspase-9 attaches to exposed caspase recognition domains (CARDS) (Yuan et al., 2010). A constant cycle of caspase-9 recognition, binding, activation, processing, and displacement is caused by the proximity of caspase-9 monomers (Malladi et al., 2009). Smac and Omi are released beforehand during MOMP and bind to and inhibit X-linked inhibitors of apoptosis (XIAP), which normally limit caspase-9 catalytic activity (Tait and Green, 2010). By using the ubiquitin proteasome pathway (UPP), XIAP causes caspases to be directly ubiquitinated and degraded (Eckelman et al., 2006).
Initiator caspase-9 and executioner caspases-3 and -7 become more readily available when XIAP is deactivated (Green and Llambi, 2015). Initiator caspase-9 then cleaves and activates executioner caspase-3 and -7, much as in the extrinsic route (Elmore, 2007). Executioner caspases then trigger cytoplasmic endonuclease and cytoskeletal peptide proteases, which may subsequently break down crucial nuclear proteins like lamin A/C and PARP (poly (ADP-ribose) polymerase), which are both catalysed by caspases 3/3/9. (caspase-6). (Green and Llambi, 2015; Wajant et al., 2002; Slee et al., 2001).
PCD of Manduca ISM:
Lockshin and William studied the loss of intersegmental muscles (ISMs) near the conclusion of metamorphosis in studies of the American silkmoth, Antheraea polyphemus. This mechanism was first identified in 1935 by Kuwana et al. and developed in 1956 by Finlayson. In order to exit their pupal cuticle at the conclusion of their pupal/adult development, adult moths use these ISMs. The next 30 hours saw the autophagic demise of these muscles (Finlayson, 1956; Beaulaton & Lockshin, 1977; Schwartz et al., 1993). The reasons behind this behaviour are still unknown, despite the fact that the phenomena of ISM loss in moths is widely understood.
The Schwartz team at UMass Amherst investigated the molecular processes behind this behaviour using the tobacco hawkmoth, Manduca sexta, as a model organism. ISM atrophy starts on day 15 of pupal development, according to research done in 1983 by Schwartz and Truman (Schwartz and Truman, 1983). The ISMs now go through hormonally-mediated atrophy, which is governed by a group of steroid hormones called ecdysteroids. During this period, they lose up to 20% of their volume and 40% of their muscular mass (Schwartz and Truman, 1983). The hormone responsible for insect moulting, 20-hydroxyecdysone (20E), was identified as the ecdysteroid in question. The expression of polyubiquitin mRNA and proteolysis both significantly rise during this phase (Schwartz et al., 1990a; Tsuji et al., 2020).
On day 17 of pupal development, a further drop in circulation levels of 20E triggers the production of the peptide hormone eclosion hormone (EH) (Schwartz and Truman; 1982). EH serves as the immediate cause of death. Rapid muscle atrophy after the release of EH is a defining feature of PCD.
(4% per hour after eclosion) mass (Schwartz and Truman 1983). Exogenous 20E administration the day before eclosion suppresses ISM PCD (Schwartz and Truman, 1984).
By showing that inhibitors of both RNA and protein synthesis might stop ISM PCD in Antheraea polyphemus, Lockshin expanded on the PCD phenomenon in 1969. (Lockshin and Williams, 1969). This provided as support for the hypothesis that de novo gene expression is necessary for PCD in silkmoths. Later, in 1990a, Schwartz et al. confirmed this. The Schwartz group was the first to clone genes linked to death by using the Manduca ISM model. Apolipophorin, 20S and 26S proteasome subunits, polyubiquitin, and other well-known compounds were among them (Schwartz et al., 1990b; Sun et al., 1995; Jones et al., 1995; Sun et al., 1997). Others, however, were brand-new proteins whose function in PCD was not yet known. This contains substances like Acheron, death-associated LIM-only protein, and small cytoplasmic leucine rich repeat protein (SCLP) (Hu et al., 1999; Kuelzer et al., 1999; Valavanis et al., 2007).
The induced sequence was dubbed Acheron (Achn) after one of the rivers of death in Greek mythology. Valavanis et al. (2007) used a differential cloning approach to discover genes that were induced or repressed when the ISMs were committed to die. When Manduca ISMs commit to dying on day 18 of pupal development, Acheron is drastically induced, according to Northern blot analysis of the mRNA expression of Acheron in Manduca ISMs (Valavanis et al., 2007). Following quantification using qPCR, it was shown that Acheron is stimulated by around 1,000 times on day 18. (Sheel et al., 2020). ISM death and Acheron expression were both slowed by 20E injection on day 17 of pupal development (Valavanis et al., 2007).
Acheron and PCD:
Acheron (LARP6), an RNA-binding protein belonging to the Lupus Antigen (LA) family with a 55kDA, is phylogenetically conserved. La-related proteins (LARPs) are RNA-binding proteins with a functional, winged helix-turn-helix structured RNA binding LA motif (LAM) and a well-conserved RNA recognition motif (RRM) (Stefanovic et al., 2014; Martino et al., 2015; Bousquet-Antonelli et al., 2009). A functional nuclear export signal (NES), subcellular localization and mRNA substrate recognition domain, and nuclear localization signal (NLS) are also present in Acheron (Valavanis et al., 2007; Stavraka et al., 2015).
Recent research by Dermit et al. indicates the significance of Acheron in the location and translation of ribosomal protein-coding mRNAs (RP-mRNAs). In order to improve Acheron localization and enrichment in actin-rich cell protrusions, Acheron also interacts with serine-threonine kinase receptor-associated protein (STRAP) through its SUZ-C domain. Here, Acheron interacts with RP- mRNAs, enhancing RP production, upregulating ribosome biogenesis, and increasing the amount of protein synthesised by migrating cells as a whole. Epithelial to Mesenchymal Transition (EMT) causes an increase in Acheron expression and subsequent protein production in human breast cancer (Dermit et al., 2020).
Intersegmental muscles (ISMs) from the moth Mnaduca sexta were used to create Acheron in its first cloning (references). Acheron expression is shown to rise 1000-fold on day 18 of pupal-adult development, when the ISMs commit to dying, using transcriptomins and qPCR (Sheel et al., 2020; Tsuji et al., 2020). When the quantity of 20E in the blood falls below a certain level in insects on day 17, the eclosion hormone receptor and the Acheron gene are both expressed (Sheel et al., 2020). On day 18, a further drop in 20E causes the release of eclosion hormone, which works on the muscles to promote the synthesis of cGMP, the transformation of inactive protein kinase-G (PKG) into active PKG (PKGa), and the phosphorylation of Acheron.
In parallel research, transcriptomics of developing Drosophila melanogaster showed that Acheron mRNA is momentarily increased by 600 times before adult eclosion (Graveley et al., 2010; Sheel et al., 2020). The results of the RNAi knockdown of Acheron expression in the muscles were muscle death before it should have, as opposed to after adult eclosion (Gurbutt et al., 2013, Sheel et al., 2020). Overall, the findings from Manduca and Drosophila point to a correlation between Acheron expression and muscle death during pupal development.
The interaction between Acheron and a pro-apoptotic BH3-only protein linked to BAD was discovered by mechanistic investigations of Acheron activity with Manduca ISM (Sheel et al., 2020). Acheron's ability to connect with a new 21 kDa BAD-like protein was revealed by Co-IP tests using day 18 ISM extracts of Acheron. When compared to Acheron, this protein's expression rose on day 18 of pupal development, according to a Western blot analysis, even though it was undetectable in the early stages of development. It's interesting to note that insects are said to lack BH3-only proteins (Nicolson et al., 2015). But computational RNA-seq study of the d18 Manduca ISM found a transcript transcribed at more than 10 RPKM that, when theoretically translated, was around 21 kDa and included patterns with similarity to BH3 domains (Sheel et al., 2020). This study led to the discovery of a new Bnip3 ortholog that shared multiple sequence regions with BAD (Sheel et al., 2020). The Schwartz Lab gave this molecule the moniker BBH-1 (BAD/Bnip-3 Homology 1). While RNAi of Acheron in the Drosophila ptelinal muscles led to the demise of valuable muscles and prevented the pharate adults from eclosing (Sheel et al., 2020).
In contrast, BBH-1 RNAi prevented muscle death, and the flies properly closed (Sheel et al., 2020). This shows that BBH-1 controls the Drosophila ecdysial muscles' ability to die directly.
Acheron interacts with the pro-apoptotic BH3-only protein, BAD, in mammalian models using Co-IP investigation of Acheron binding partners in C2C12 myoblasts, a presumed satellite cell line generated from adult mouse muscle and a common cell line used for studying myogenesis (Sheel et al., 2020).
Acheron binds to the ubiquitin ligase Human Homolog of Ariadne-1 (HHARI), according to co-IPs and yeast 2-hybrid screening (Wang et al., unpublished). The Parkin ubiquitin ligase, a recognised protein associated with autosomal recessive diseases, has no known near homolog, although HHARI does.
parkinsonism) (Aguilera et al., 2000). (Aguilera et al., 2000). Both proteins bind to the same ubiquitin conjugases (UbcH7 and UBcH8) and have comparable RING (Really Interesting New Gene) finger domains in common (Moynihan et al., 1999). Together, these findings imply that HHARI may serve as a ubiquitin ligase comparable to Parkin and be involved in controlling protein turnover (Marin and Ferrus, 2002).
Together, these results provide credence to the idea that Acheron's capacity to bind to poisonous BH3-only proteins (BAD in mammals and BBH-1 in insects) is a key component of its survival function (Sheel et al., 2020). The new pro-apoptotic protein BBH-1 is bound to by Acheron, which subsequently sequesters it, causing it to accumulate. Then, phosphorylated Acheron is likely destroyed by the Ubiquitin Proteasome Pathway (UPP), releasing BBH-1 in the process. Inhibiting or avoiding the activation of the anti-apoptotic Bcl-2 proteins, BBH-1 is subsequently thought to activate PCD via the intrinsic cell death pathway, resulting in MOMP (Sheel et al., 2020, Delbridge et al., 2016).
Acheron and Myogenesis:
The multifunctional protein Acheron takes involved in several developmental stages. The theory that Acheron is an essential regulatory molecule in the differentiation of both ciliated cells and muscles in mammals is supported by published research (Wang et al., 2009; Glenn et al., 2010; Manojlovic et al., 2017). In order to verify the idea that Acheron is essential for myogenesis, Wang et al. carried out immunofluorescence (IF), immunocytochemistry (ICC), Western blot, and caspase tests in both C2C12 myoblasts and zebrafish embryos in 2009. These studies' findings reinforced the idea that Acheron expression in C2C12 cells is developmentally controlled and that Acheron is a crucial component of myogenesis and may even control the survival of stem cells that are limited to the skeletal muscle. The aforementioned results also provide credence to the theories that Acheron controls the development of fast and slow twitch fibres in living cells and that Acheron causes reserve cells to die during differentiation.
The mouse cell line C2C12 is one of the model systems that has proven beneficial for analysing the expression and function of Acheron. These myoblasts are capable of committing to one of three probable destinies when they aren't given growth stimuli (Yaffe and Saxel, 1977). A minority of cells that follow the first route express the beta helix-loop-helix (bHLH) transcription factor MyoD (myoblast determination protein). This cell cycle arrest, myogenin expression, and differentiation into multinucleated myotubes are all results of this arrest (Thayer et al., 1989; Tapscott, 2005).
These cells' expression of the bHLH protein Myf5 opens up the second potential route (Myogenic factor 5). Myoblasts leave the cell cycle and arrest in G0 when Myf5 is expressed. Additionally, these cells increase their production of Bcl-2, an anti-apoptotic protein, making them more apoptosis-resistant (Dominov et al., 1998, 2001; Yoshida et al., 1998). Finally, a portion of cells fail to end the cell cycle or produce myotubes, and instead undergo apoptosis (Dee et al., 2002).
Acheron expression in C2C12 cells was shown by Wang et al. to be developmentally controlled (Wang et al., 2009). MyoD, a nuclear phosphoprotein that promotes the growth of multinucleated myotubes and drives myogenesis, was discovered by Western blot analysis of cycling and differentiated C2C12 cells (Tapscott et al., 1988; Wang et al., 2009). MyoD-expressing cells activate the lineage markers MyoA and MyoH when they lack growth factors. Proliferating myoblasts acquire differentiation signals from nearby cells after growth factors have been completely removed or exhausted. Acheron plays a crucial function in myogenesis by acting upstream of the muscle-specific transcription factor MyoD. Exogenous Acheron transfection caused cells to develop thicker muscle fibres with more nuclei and showed an almost total loss of satellite cells from the culture. Antisense Acheron transfected cells showed reduced myosin heavy chain (MHC), Myf5, and MyoD levels (Wang et al., 2009). An N-terminally shortened version of Acheron (tAchn), which seems to act as a dominant-negative regulator, was transfected into cells, and Western blot analysis showed that these cells produce MHC but not MyoD, high levels of Myf5, which indicates cellular arrest in G0 (Wang et al., 2009). Together, these results provide evidence for the hypothesis that Acheron is essential for myogenesis and cellular differentiation, and that blocking Acheron expression or activity prevents myogenesis.
Glenn et al. used immunofluorescence (IF) and migration tests using C2C12 myoblasts and myotubes to obtain insight into the molecular processes underlying Acheron's function in controlling myogenesis (Glenn et al., 2010). After being transfected with a plasmid encoding the full-length sequence of Acheron (FL-Achn), the transient activator of transcription (tAchn), or the antisense form of Acheron (AS-Achn), cells were examined for the presence of myogenesis-related proteins like MHC and both the A and B isoforms of the integrin subunit specific to laminin, 7A and 7B. Cells undergoing myogenesis need differentiation signals given by surrounding cells and the extracellular matrix in order to differentiate. Integrins are transmembrane proteins that control interactions (Boettiger et al., 1995).
After being transferred to differentiation medium (DM), cells expressing ectopic AS-Achn or tAchn prevented the production of 7A e, but cells expressing ectopic Acheron produce higher quantities of these Acheron proteins by the third day in DM (Glenn et al., 2010). As opposed to ASAchn- and tAchn-expressing cells, control and Achn-expressing samples were shown to have higher baseline levels of 7B protein and mRNA. In Achn-expressing and control samples after transfer to DM, 7B expression increased while remaining constant in ASAchn- and tAchn-expressing cells (Glenn et al., 2010). Myogenesis is increasing, while ITGB1, a crucial molecule in controlling cellular adhesion, is being downregulated, according to analysis of Acheron expression (Glenn et al., 2010). A migratory phase that peaks 24 hours after transfer to modified cells is indicated by increased regulation of 7A and 7B in the hours that follow (Glenn et al., 2010). An essential stage in cellular proliferation and adherence to extracellular matrix components is improved as a consequence, increasing cell to matrix contacts and adhesion differentiation (Beacham et al., 2006). (Beacham et al., 2006). When considered together, these data provide plausible methods for how Acheron-regulated myogenesis is mediated.
Hau et al. depleted Acheron mRNA from Xenopus embryos using morpholino oligos, much as the Schwartz lab's earlier zebrafish investigation. Following that, samples were examined using whole mount in situ hybridization and RT-PCR (WISH). These investigations' findings demonstrated that basal bodies at centrioles, which are necessary for ciliary assembly, were downregulated in the presence of Acheron knockdown, resulting in abnormalities in neural tube closure and a lack of cilia in the epidermis. Additionally, according to these scientists, Acheron regulates the expression of DNA synthesis-associated cell cycle protein (mcidas) and multicillate differentiation in a Notch-independent way. In Acheron knockdown cells, Mcidas stimulates the transcription of genes involved in centriole formation and may be utilised to partly restore multi-ciliated cell differentiation (Hau et al., 2020). These results provide credence to the idea that Acheron participates not only in myogenesis but also in the differentiation of ciliated cells.
Acheron and Pathogenesis:
A variety of illnesses, including cancer, muscle and neurodegenerative disorders, and AIDS, are linked to PCD dysregulation (Nagata et al., 2017; Reed et al., 1999). Since the three membrane growth factor receptors, ER, PR, and HER2/neu, which are the conventional targets for therapeutic intervention, are absent from triple negative basal-type breast cancer (TNBC), the prognosis for patients with TNBC is dismal (William et al., 2010). TNBC cell lines like MDA-MB-231 and Hs578T are often employed as cancer research models. Acheron expression was discovered to be higher in basal-type breast tumours compared to controls using microarray analysis and immunohistochemical tests on breast cancer tissues (Shao et al., 2012).
When implanted into immunodeficient SCID mice, MDA-MB-231 cells altered to produce ectopic Acheron developed tumours that were 5X bigger than non-engineered MDA-MB-231 controls (Shao et al., 2012). Additionally, ectopic Acheron-expressing tumours had higher levels of vascularization. This apparently caused proliferation to occur without being constrained by typical tumour development restrictions such food shortage and oxygen diffusion. Increased expression of the proteins matrix metalloproteinase 9 (MMP-9) and vascular endothelial growth factor were also seen in cells expressing Acheron, along with increased invasiveness through Matrigel (VEGF) (2012) Shao et al.
MMPs are created from inert zymogens. MMPs become active and break down the extracellular matrix (ECM) once the pro-domain or residue modification is removed by proteolysis (Kessenbrock et al., 2010). This behaviour is physiologically controlled by factors including immunological reactions and inflammation (Kessenbrock et al., 2010). After activation, MMPs degrade the ECM around tumours that are actively growing. Due to the resulting cell migration and subsequent inflammation, a positive feedback loop to release additional MMPs is set off. MMPs are one of the primary indicators of aggressive malignancies as a result. When compared to wild type cell lines, MDA-MB-231 cells with MMP-9 knockdown (KD) showed significantly reduced levels of Matrigel invasion. Tumors with decreased MMP9 expression developed when transplanted into mice, however unlike WT tumours, these tumours were unable to spread to the animals' lungs (Mehner et al., 2014).
By causing the release of activated vascular endothelial growth factor-A (VEGF-A) and vascular endothelial growth factor receptor 2 (VEGFR-2), MMP-9 differs from other MMPs in that it further improves angiogenic activity (Shibuya et al., 2011). By attaching to VEGFR-2, VEGF-A promotes the proliferation of endothelial cells. As a result, tumour vascularization, tumour growth, and cell proliferation are all improved. Three human cancer indicators that significantly enhance disease mortality (Wang et al., 2018).
TNBCs have a greater capacity for invasiveness and angiogenesis.
Human ductal carcinoma immunohistochemical labelling demonstrates that Acheron expression is increased in malignant tumours that are aggressive (Shao et al., 2012). TNBCs with ectopic Acheron expression have increased invasiveness and angiogenic capacity. Together, these facts provide credence to the idea that Acheron is essential for the pathogenesis of TNBC.
The pathophysiology of the liver may also be influenced by Acheron dysregulation. The illness fibrosis is characterised by increased amounts of type I collagen in different organs (Ghosh et al., 2002).
Acheron regulates the subcellular location and expression of type I collagen mRNAs by binding to their 5' stem loop (5'SL) (Cai et al., 2010). Loop 3 of Acheron's RNA Recognition Motif (RRM) binds to the 5' SL and sequesters the complex to proteins like the protein transport protein SEC61 translocon, causing this to happen (Stefanovic et al., 2014). Following the C-terminal phosphorylation of Acheron, type I collagen production takes place. At the C-terminus, there are six serine residues, all of which are phosphorylated in a hierarchical manner to promote the production of type I collagen. This starts with the phosphorylation of S451 by Protein Kinase B (Akt/PKB), and continues with the phosphorylation of S348 and S409 by the Mammalian Targets of Rapamycin Complex 1 (mTORC1). Type I collagen production is hindered or decreased as a consequence of improper control of phosphorylation at these locations or suppression of Akt and mTORC1, respectively (Zhang et al., 2016, 2017). Immunosuppressive medications like Tacrolimus (FK506) that disrupt the Acheron/collagen mRNA binding restrict the Acheron to 5'SL binding and significantly lower the production of type I collagen (Manojlovic et al., 2013).
A dynamic protein known as acheron, it is essential for PCD, myogenesis, malignancy, and hepatic fibrosis. Acheron has functions in additional illnesses that have not yet been fully investigated, despite the fact that these two disease pathways have been identified. A characteristic of type 2 diabetes' pathogenesis, greater proinsulin levels are also linked to acheron (Strawbridge et al., 2011).
By examining Acheron's capabilities to serve as an RNA binding and signal transduction protein, it becomes clearer how it may participate in a variety of crucial physiological activities. The multidomain scaffolding protein calcium/calmodulin-dependent serine protein kinase 3 (CASK) may influence ion channels, receptors, and cell adhesion molecules in epithelial tissues, motor neurons, and muscles to help regulate signalling (Dimitratos et al., 1998). CASK binds to the C-terminus of Acheron through its CaM K II-like domain, according to a yeast 2 hybrid screening experiment using Acheron as the bait and a mouse embryonic cDNA library as the prey (Weng et al., 2009). The complexation of CASK and Acheron with transcriptional repressors of the helix-loop-helix (HLH) family known as Id (inhibitor of DNA binding/differentiation) allows for the control of gene expression (Hsueh, 2006; Weng et al., 2009).
Through RNA and protein-protein interaction, Acheron can operate as a crucial regulatory molecule thanks to its functioning RRM, La Motif, and RBD. Acheron binds to the 5' stem-loop structure (SL) of type I collagen mRNA to control the production of type I collagen. Translation of the collagen mRNA is enabled by Acheron's binding to the 5'SL (Fritz et al., 2011). When structural proteins like Myosin IIB and Vimentin join forces to create the Acheron complex, it binds to the 5'SL of type I collagen and sequesters it to the ER membrane (Wang et al., 2014; Cai et al., 2010; Manojlovic et al., 2012). Then, complexed Acheron attaches to the C-termini of RNA Helicase A (RHA) and Serine-Threonine Kinase Receptor-Associated Protein (STRAP), enabling translation (Vukmirovic, 2013; Manojlovic et al., 2012).
A dynamic chemical called acheron has important regulatory roles in PCD, myogenesis, and pathogenesis. Acheron action is governed by protein-protein interactions and its subcellular localization patterns, according to a number of lines of evidence, despite the fact that the mechanisms governing its action are poorly understood. In this dissertation, I will use immunofluorescence and confocal microscopy to carry out two studies that may shed light on Acheron's functions in mammalian models of programmed cell death, differentiation, and pathogenesis in addition to revealing its subcellular distribution and potential binding partners.
It is generally known that comprehending a protein's subcellular localization may assist explain both the structure of the cell as a whole and the function of an individual molecule (Scott et al., 2005). A dynamic protein, Acheron plays mechanistic roles in processes within and outside the nucleus, such as differentiation, programmed cell death, and disease, respectively. It has functional nuclear localization and nuclear export signals (Valavanis et al., 2007, Sheel et al., 2020). Acheron's precise subcellular location remains uncertain, nevertheless. I initially investigated the idea that Acheron localises to mitochondria because it interacts with BAD, a protein directly connected to MOMP.
Protein-protein interactions may have a role in Acheron's participation in PCD, myogenesis, and pathogenesis, according to available evidence. I'll investigate the claim that the protein Acheron colocalizes with the molecules BAD, HHARI, and calcium/calmodulin-dependent Serine Protein Kinase (CASK).
CHAPTER II
DETERMINING THE LOCALIZATION OF ACHERON AND SPECIFIC BINDING PARTNERS IN A MAMMALIAN SYSTEM
Introduction:
The multifunctional protein Acheron possesses various protein-protein interaction motifs that are likely responsible for many of its biological functions. Even while new information on the subcellular processes that control Acheron function has emerged, there are still many unanswered questions.
.png)
.png)
Table 1a. Known Acheron Binding Partner Roles, Localization and Evidence:
Listed here is a list of recognised Acheron binding partners with established involvement in PCD, myogenesis, and pathogenesis. The location of each molecule and the information available describing its interaction with Acheron are also given in the table along with each molecule's physiological function.
I used an anatomical approach to test three main assumptions in order to understand some of the molecular processes underlying Acheron function in mammalian systems. The first investigation established Acheron's subcellular location in mammalian cells. Here, I assessed how well Acheron adhered to the various mitochondria, actin filaments, and microtubules. To identify Acheron's cellular location, cycling mononucleotide C2C12 murine myoblasts, differentiated C2C12 my tubes, and U2-OS osteosarcoma cells were immunostained. The second research examined the idea that Acheron's binding partners' homologs locate alongside Acheron in mammalian cells. In this investigation, HHARI, BAD, and CASK in relation to -tubulin were stained on Wild-type C2C12 myoblasts and U2-OS osteosarcoma cells. The third and final experiment explored the possibility that CASK moves to the nucleus at the same time that Acheron does in response to growth factor stimulation. To test this theory, C2C12 cells were cultivated on glass coverslips with ectopic full-length GFP-tagged Acheron and C2C12 cells with ectopic full-length GFP-tagged Acheron that had deletions in their nuclear export signal (- NES). High concentrations of growth stimulants were then used to push Acheron into the nucleus, where it was trapped in -NES cells. The position of Acheron and CASK in regard to microtubules was then labelled in cells, and any translocation was examined.
Materials and Methods
C2C12 Cell Culture:
In DMEM (Dulbecco's Modified Eagle Medium) + 10% FBS (Fetal Bovine Serum) + 1% penicillin/streptomycin in 10% CO2, C2C12 cells were cultured at 37°C. A new growth media was made by vacuum filtration in a tissue culture hood at 25°C using 445 mL DMEM pH 7.2, 50 mL FBS, and 5 mL Pen/Strep. The cells were then taken out of liquid nitrogen storage and promptly defrosted by rotating the tube in a water bath that was maintained at 37°C. Vials were transferred to 15 mL conical tubes with 5 mL of full C2C12 DMEM media after being 70% thawed, sterilised with ethanol, and then centrifuged at 500 x G for 5 minutes.
After discarding the supernatant, the pellets were reconstituted in 10 mL of full C2C12 DMEM media and then plated on 10 cm plates with flat bottoms. After that, cells were divided every 48 hours (or when they achieved around 70% confluency) by aspirating the medium and twice rinsing them in PBS (phosphate buffered saline). The cells were then added to 0.53mM EDTA + 0.25% (w/v) trypsin that had been warmed to 37°C. Cells were once again transferred to a 15mL conical after being removed from the dish's bottom and centrifuged at 500 x G for 5 minutes. The pellets were then resuspended in 10 mL of full C2C12 DMEM media after the supernatant was discarded. Then, cells were plated in a 10 cm flat-bottom dish at a ratio of 1:10 of cells to new media.
C2C12 Differentiation:
C2C12 cells were cultivated to a confluency of around 70% before being moved to a low serum differentiation medium (DM) composed of DMEM + 1% Pen/Strep + 2% horse serum. The differentiation medium was changed every 48 hours while the cells were cultured in it for 5 days at 10% CO2 37°C. For effective differentiation, the development of myotubes in the cells was seen under a microscope.
Glass Coverslip Coating Removal and Poly-L-Lysine Treatment:
Glass coverslips (Fisher Scientific) were treated for 15 minutes with 21 mM Poly-L-Lysine (Sigma Aldrich) to improve cell-to-coverslip adhesion. Coverslips were taken out of their packing, put on the shaker, and spun at 100 rpm for six hours at 25 °C in 25 mL of 2M NaOH.
After that, coverslips were submerged in 20 mL of dH2O and shaken for a whole night at 25 °C at 150 rpm. The next day, coverslips were washed in running dH2O before being sterilised in the autoclave. After being washed with 70% ethanol, sterile coverslips were positioned in the tissue culture hood. Coverslips were coated with 10 mL of 21 mM Poly-L- Lysine and left in the tissue culture hood for 15 min at 25 °C. Coverslips were then rinsed with Poly-L-Lysine solution, aspirated, and overnight air dried in the tissue culture hood with the filtration on. The resulting coverslips were deemed sterile and suitable for cell culture.
C2C12 + HA-Acheron -NES / +NES Mutant Acheron Translocation:
For the studies, ectopic Acheron with a HA-tag was expressed in C2C12 cell lines. Cycling C2C12 cells were transfected with a plasmid that used a CMV promoter to drive ectopic Acheron expression. The Nuclear Localization Signal, NLS (-NLS), or the Nuclear Export Signal, NES (-NES), were then deleted from constructs using site-directed mutagenesis and injected into cells (Shao et al., 2012). We used mutant cells with malfunctioning NESs and cultivated them at high growth serum to "push" Acheron towards the nucleus in order to ascertain Acheron's function there (Glenn and Schwartz, unpublished). The cells were grown in conventional C2C12 growth media (10% FBS in DMEM) for 24 hours on glass coverslips at 37 °C and 10% CO2. The cells were then transferred back to high growth serum medium (15% FBS) for 2 hours after spending the previous night in 3 mL DMEM + 1% Pen/Strep + 2% horse serum. The medium was aspirated once the cells were taken out of the incubator. Following a 1X PBS washing, the cells were fixed by adding 2 mL of 4% paraformaldehyde to 1X PBS at 4°C and incubated for 10 to 15 minutes at 25°C.
Paraformaldehyde was fixed, then removed and disposed of as hazardous waste. Dishes were sealed with parafilm and coated with 1X PBS to keep them at 4°C until they were ready for immunofluorescence staining.
Nocodazole Mediated Microtubule Depolymerization:
Nocodazole (Sigma Aldrich), an anti-cancer drug, was used to treat C2C12 cells in order to cause microtubule depolymerization and sabotage connections with microtubule-associated proteins (MAP) (Verstraelen et al., 2017). C2C12 cells were grown on glass coverslips coated with Poly-L-lysine in flat bottomed 6-well plates in GM at 37 °C with 10% CO2. The media was aspirated and new growth medium was added after the cells reached 70% confluence. Then, nocodazole was immediately added to each well, and it was incubated for an hour at 37°C with a final concentration of 500nM. Figures 6a and b show the empirical results of the concentration determination.
C2C12 and U2OS: Acheron, BAD, Ariadne, CASK, E7 Tubulin Colocalization
Immunofluorescence Staining:
C2C12 cells were stained after being cultivated in GM at 37 °C and 10% CO2 on glass coverslips coated with poly-L-lysine. Dishes were taken out of the incubator once the cells reached the required confluency and surface sterilised with 70% ethanol. After that, dishes were brought into the tissue culture hood. Each well's remaining medium was aspirated, and the cells were then washed with 1X PBS pH 7.2 until the medium's red/pink colour was gone. Then, after being incubated at 25°C for 10-15 minutes, the cells were fixed by adding 2 mL of 4% paraformaldehyde pH 7.2 in 1X PBS at 4°C. Dishes were sealed with parafilm and coated with 1X PBS to keep them at 4°C until they were ready for immunofluorescence staining.
Samples were taken out of the refrigerator and given a fresh 1X PBS pH 7.2 wash at 25°C for 5 minutes before to the immunofluorescence staining. Then, slides were put via a to improve fluorescent signal and reduce autofluorescence, methanol permeabilization step is used. 100% methanol was stored overnight at -20°C. After being cleaned, slides were put on ice and coated with 100% ice-cold methanol. To avoid dehydration, coverslips were immediately put to -20°C for 8 minutes. Existing methanol was eliminated from each slide after 8 minutes. After that, slides were washed on the shaker at 300 rpm for 5 minutes while being submerged in brand-new 1X PBS at 25°C.
Coverslips containing cells were first permeabilized with methanol, washed, and then incubated in an immunofluorescence blocking solution. During the methanol permeabilization process, blocking solution was freshly prepared by combining 5% goat serum (ThermoFisher Scientific) in 1X PBS at 25°C and mixing by inversion. Following that, coverslips were incubated in 1 mL of immunofluorescence blocking solution overnight at 4 °C with 200 rpm of the shaker. Parafilm was also used to wrap dishes at this period to stop slides from drying out.
The next day, each coverslip received a 1mL fresh primary antibody dilution solution in lieu of the blocking solution. We prepared the primary antibody dilution solution by mixing 2% BSA (Sigma Aldrich) diluted in 1X PBS at 25°C with 1X PBS pH 7.2. The appropriate primary antibodies were then applied to the appropriate slides at the following dilutions, and the slides were shaken at 200 rpm at 4°C overnight to incubate them. To avoid coverslip dehydration, plates were once again covered with parafilm during this period.
1. E7 (DSHB) Anti-Beta Tubulin, 1:350 l
2. LARP6 (Schwartz Lab Purified) Anti-Acheron, 1:1000 mL.
3. Anti-Ariadne: 1:5000L of HHARI1 (Schwartz Lab Purified).
4. Anti-BAD and BAD (Abcepta) - 1:500 l
5. Anti-BAD and BAD (LSBio), 1:500 l
6. CASK (ABGENT) - 1:500L Anti-CASK C-Terminus
After incubation, dilution solution was withdrawn from each well, and slides were then washed three times in 1X PBS pH 7.2 at 25°C for 20 minutes while being shaken at 350 rpm. Fluorescent dyes were then applied to each individual slide at the following dilutions after the slides had been coated with 1mL of fresh 1X PBS pH 7.2. After that, slides were hidden from view and incubated in PBS/fluorescent dye solutions for 1.5 hours at 25°C.
1. Nuclear stain DAPI, 1:300 mL
2. Dylight 594-1:500L
3. Alexafluor 647, 300 L
4. Alexafluor 488 - 0.5 mL
5. Mitotracker CMXRos 40 m
6. 1:1000 L Rhodamine Phalloidin
Following the removal of the secondary antibody solutions from each slide, the slides were once again washed three times on the shaker at 350 rpm in 1X PBS pH 7.2 at 25 °C for 20 minutes. After that, coverslips were taken out of the wells and mounted using 6.5 l of immunofluorescence mounting media face down on labelled slides (4:1 glycerol in PBS). Before imaging, slides were allowed to dry overnight at 4°C with the edges sealed with clear nail polish to avoid coverslip dehydration. After that, slides were processed in Fiji and photographed using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope.
Results
Acheron Localization in Mammalian Cells:
Cycling murine C2C12 myoblasts, differentiated C2C12 myotubes, and human U2-OS osteosarcoma cells were treated for immunofluorescence labelling of Acheron in order to ascertain the subcellular location of this protein in mammalian cells. Areas of colocalization between Acheron (green) and other markers (actin, mitochondria, and tubulin) (red) may be deduced from the resultant yellow signal throughout these tests.
Co-IP of Acheron in C2C12 myoblasts revealed binding to the BH3-only protein BAD, which is typically found localised in mitochondria (Sheel et al., 2020). (Jiang et al., 2006). Mononucleated C2C12 myoblasts were stained for Acheron (green), nuclei (DAPI (4′, 6-diamidino-2-phenylindole/DNA fluorescent dye) (blue), and mitochondria (Mitotracker CMXRos) (red), and imaged at 60X on a Nikon Eclipse TE2000 inverted fluorescent microscope to test the hypothesis that Acheron localises to mitochondria with BAD in some circumstances. The bulk of the Acheron does not colocalize with the mitochondria, despite the fact that tiny quantities of yellow signal may be observed in the cell's perinuclear regions (Figs 1a, b). Instead, the cytoskeleton and nucleus seem to be the primary sites of Acheron expression. The fluorescence intensity in arbitrary units (a.u) is shown versus the cell's diagonal length in Figure 1b (microns). Simply put, this graph shows how bright each pixel is in relation to other parts of the cell. The Acheron and Mitotracker intensities are shown as green and red lines, respectively, while DNA staining is shown as the blue line. The cell nucleus may be precisely identified by the increase in DAPI (blue) fluorescence. This picture thus shows us where the majority of the Acheron is located inside the cell (Figure 1a). Acheron (green) and Mitotracker (red) peak may be detected outside the nucleus in the graphic (blue). Our perinuclear colocalizing signal, most likely. Areas of DAPI fluorescence may be observed overlapping with a large portion of the remaining Acheron signal (blue).
This shows that a significant portion of the Acheron dwells in the nucleus of this cell, whereas the other components are either present perinuclearly or in the cytoplasm of the cell (Figure 1b). The staining seen in Figure 1c lends credence to this. Acheron staining with higher resolution reveals that the cytoplasmic, non-nuclear Acheron signal is not uniform but rather seems to follow a particular cytoskeletal structure (Figure 1c). We can discern an unique reticulated pattern in our perinuclear Acheron signal thanks to the improved clarity of the Acheron staining in this Figure. Along the border of the nucleus, Acheron concentrations may be detected. Acheron may also be observed moving in a specific pattern away from the inner membrane but still close to the nuclear envelope's edge and farther inside the cell. These are probably the rough endoplasmic reticulum and the perinuclear region, respectively. Small Acheron aggregates may also be detected at the cell's edge, however they are clearly on protrusions that stick out from the cell rather than the leading edge. This most likely affects filopodia rather than lamellipodium (Figure 1c). This gave us the impression that Acheron was concentrating in the cytoskeletal components of the cell.
Figure 1a.: Subcellular Localization of Acheron relative to Mitochondria in C2C12 Myoblasts
A 60X z-projection of a single stained cycling mononucleotide C2C12 myoblast's nucleus (DAPI; blue; far left), mitochondria (Mitotracker; red; second to the left), Acheron (anti-Acheron; green; second to the right), and composite (merge; far right) channels. Some
While the majority may be seen in the cell's nucleus or cytoplasm, Acheron can be observed localizing with mitochondria in the yellow sections of the picture.
Figure 1b. Colocalization analysis of DAPI, Acheron and Mitochondria fluorescent signal from figure 1a.
An illustration of the relationship between fluorescence intensity for DAPI(blue), mitochondria (red), and anti-Acheron (green) and cell area. However, substantially more of the Acheron signal can be seen overlapping with the DAPI (blue) signal at the cell's nucleus and in its cytoplasm. Acheron (green) fluorescence intensity can be seen overlapping with mitotracker (red) in areas of perinuclear signal.
Figure 1c. Acheron Staining Follows a Cytoskeletal Pattern in C2C12 Myoblasts.Figure 1a's Acheron (green) staining as seen up close. Instead of being diffuse, the cytoplasmic signal seems to follow a cytoskeletal structure.
Cycling C2C12 myoblasts were labelled for Acheron (green), DNA (DAPI; blue), and actin (Rhodamine Phalloidin; red) and photographed at 60X using a Nikon Eclipse TE2000 inverted fluorescence microscope to test the theory that Acheron binds to actin filaments (Figure 2a). In this image, there are no colocalization regions (yellow), which suggests that Acheron does not bind to actin filaments. However, Acheron is once again detected accumulating towards the actin filament edges (likely the filopodia). Additionally, the acheron signal is more diffuse in the nucleus and the region around it, but pattern-like in the cytoplasm, suggesting that there may be various molecular actions depending on protein location (Figure 2a). The information from Figure 2a's graphs enables one to reach the same conclusions. Rhodamine phalloidin (red) fluorescence and Acheron (green) signal both exhibit cytoskeletal expression patterns that are comparable, but the signals do not coincide, indicating that Acheron (green) does not localise to Actin (red) filaments in the cell body. However, similar to Figure 1b, Figure 2b also exhibits a spike in Acheron (green) expression at the periphery. Probably, Acheron's enriched region is located here.
Confined to the cell's filopodia. Even while this confirms the findings discovered by Dermit et al., the idea that Acheron attaches to actin filaments is not supported by the lack of yellow fluorescence (Figure 2b). As a result, we focused on microtubules, another cytoskeletal component.
Figure 2a: Subcellular Localization of Acheron Relative to Actin Filaments in C2C12 Myoblasts.
A 60X z-projection showing a single stained cycling mononucleated C2C12 myoblast's nucleus (DAPI; blue; far left), actin filaments (Rhodamine Phalloidin; red; second to the left), Acheron (anti-Acheron; green; second to the right), and composite (merge; far right) channels. Acheron may not bind to actin filaments as there are no regions of apparent colocalization (denoted in yellow). Acheron may still be recognised, nevertheless, in the cytoplasm and nucleus in a cytoskeletal pattern.
Figure 2b. Colocalization analysis of DAPI, Acheron and Rhodamine Phalloidin Fluorescent Signal From Figure 2a.
A graphical representation of fluorescence intensity for DAPI (blue), Rhodamine Phalloidin (red) and Anti-Acheron (green) plotted relative to area on the cell. Acheron (green) fluorescence can be seen following a separate staining pattern from Rhodamine Phalloidin signal (red).
We stained cycling C2C12 myoblasts for Acheron (green), DNA (blue), and -tubulin (red) to examine the possibility that Acheron attaches to microtubules (Figure 3a). Microtubule formations are made up of the tubulin monomers -tubulin and -tubulin, which polymerize together (Gunning et al., 2015). A significant amount of overlap was found between the Acheron and -tubulin signals (yellow), indicating that the bulk of the Acheron protein (green) may be seen colocalizing with -tubulin (red) on microtubules (Figure 3a). Similar to earlier Acheron labelling, green, fluorescent signal is seen at cell protrusions, which are probably actin-rich filopodia. In Figure 3b, the precise level of colocalization is visually shown. This graph also shows fluorescence intensity (a.u.) graphed versus the diagonal length of the cell, similar to Figure 1b (microns). The blue line in this diagram represents DNA, whereas the green and red lines, respectively, in this diagram indicate Acheron and -tubulin. even when the DNA signal is the Acheron and -tubulin signals localised to the nucleus of the cell are detected fluorescing at different intensities but at roughly the same places throughout the cell.
One region outside the nucleus also experiences an increase in acheron and -tubulin signal (Figure 3b). This is most likely the centrosome, a cellular component essential to microtubule organisation (Bornens and Azimzadeh, 2007). The aberrant spikes of Acheron (green) signal at the cell's edge serve as a visual representation of the Acheron signal aggregates found in cell protrusions. Together, these findings lend credence to the idea that Acheron localises to microtubules.
Rather than using a Nikon Eclipse TE2000 inverted fluorescent microscope, cycling C2C12 myoblasts were labelled for Acheron (green), DNA (blue), and -tubulin (red) and photographed at 100X using a Nikon A1R- SIMe resonant scanning confocal super resolution microscope (Figure 3c). Confocal microscopy employs a spatial pinhole to exclude out-of-focus light during image capture, which sets it apart from traditional inverted fluorescence microscopy. The optical resolution and contrast of microscopic samples are greatly improved. Scanning confocal microscopes may also automatically take a number of two-dimensional pictures within a predetermined focus range. The "z-slices" that are produced may be used to recreate a three-dimensional structure. When two proteins are believed to be simultaneously occupying the same location, these methods become crucial for co-localization research. Precision co-localization analysis is made possible by the ability to conduct protein localization analysis in z-slices up to 0.125 microns thick (Pawley, 2006; Jensen, 2004). Five 0.125-micron thick z-slices, or a roughly 0.625-micron thick z-stack, were taken from the three-dimensional reconstruction of this picture and processed separately. All of the microtubule and acheron in-focus slices are present in this region of the cell, free from interference from the optical slices above or below. Because of the removal of optical "contamination," resolution is improved (Figure 3c). Again, extensive areas of yellow fluorescence throughout the cell indicate colocalization of both proteins.
Returning to the centrosome to assemble. Additionally, similar to earlier photos, strong clusters of green (Acheron) fluorescence can be detected along the cell's edge. This provides further evidence in favour of our claim that Acheron binds to microtubules. At the edge of the cell, patches of distinctly red microtubules can also be observed in this view. We are not given this level of detail by conventional inverted fluorescence microscopy.
When the same information is again examined visually, it becomes even more clear. Pictures obtained using confocal super resolution microscopy include thousands of data points, enhancing the temporal resolution compared to images obtained using ordinary inverted fluorescence microscopy, which only have hundreds of data points. Here, the Acheron (green) and -tubulin (red) signals perfectly match each other. Additionally, the Acheron signal is unaffected by peaks in the DAPI (blue) fluorescence (Figure 3d).
Figure 3a. Subcellular Localization of Acheron Relative to Microtubules in C2C12 Myoblasts.
A z-projection of the stained cycling mononucleated C2C12 myoblasts' nuclei (DAPI; blue; far left), microtubules (E7; red; second from the left), Acheron (anti-Acheron; green; second from the right), and composite (merge; far right) channels is shown here. Smaller concentrations of Acheron are shown localising to the cell's nucleus, while larger concentrations are visible colocalizing with microtubules in the yellow portions of the picture and becoming more intense near the cell's centrosome. This suggests that Acheron and -tubulin have a high colocalization.
Figure 3b. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal From Figure 3a.
DAPI (blue), -tubulin (red), and Anti-Acheron (green) fluorescence intensity is shown on a graph in relation to cell surface area. Acheron's (green) and -tubulin's (red) fluorescence intensities nearly exactly overlap, showing that both proteins may be detected on the same regions of the cell while having differing levels of expression.
Figure 3c. Super Resolution Subcellular Localization of Acheron Relative to Microtubules in C2C12 Myoblasts.
A 100X confocal z-stack of a single labelled cycling mononucleated C2C12 myoblast's nucleus (DAPI; blue; far left), microtubules (E7; red; second to the left), Acheron (anti-Acheron; green; second to the right), and composite (merge; far right) channels. A little amount of Acheron can be detected in the cell's nucleus, but the majority of it can be observed colocalizing with microtubules in the yellow portions of the picture, with greater patches of yellow fluorescence in the cell's centrosome.
Figure 3d. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal From Figure 3c.
DAPI (blue), -tubulin (red), and Anti-Acheron (green) fluorescence intensity is shown on a graph in relation to cell surface area. While DAPI fluorescence is still largely distinct, the intensity of the anti-Acheron (green) and -tubulin (red) fluorescence nearly fully overlaps.
We stimulated myotube formation in cycling C2C12 myoblasts to test the theory that Acheron binds also to microtubules in differentiated skeletal muscle. DNA (blue), -tubulin, and Acheron were used to stain myotubes (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a magnification of 60X. (Figure 4a). Myoblasts are shown in this figure as mononucleated cells, while myotubes are represented as multi-nucleated cells, demonstrating complete differentiation of each tube. Here, colocalization is shown by the appearance of Acheron in yellow areas of the picture with -tubulin. From Figures 3a and 3c, there is also a noticeable rise in acheron fluorescence (and hence, expression). This supports earlier findings that distinct C2C12 myotubes express more Acheron.
In addition, confirms our theory that in differentiated myotubes, Acheron binds to microtubules (Figure 4a). We once again conducted a visual analysis of the photographs to better clarify these conclusions.
The DAPI signal is shown as the blue line in this graph, whereas Acheron and -tubulin are shown as the green and red lines, respectively, in Figures 3b and 3d (Figure 4b). Our multi-nucleated myotube's numerous nuclei are indicated by a number of blue peaks. Once again, the expression patterns of acheron (green) and -tubulin (red) are essentially identical (Figure 4b). This strengthens our claim that differentiated C2C12 myotubes of Acheron bind microtubules.
Figure 4a. Super Resolution Subcellular Localization of Acheron Relative to Microtubules in C2C12 Myotubes.
A 60X confocal z-stack of a single labelled cycling mononucleated C2C12 myoblast's nucleus (DAPI; blue; far left), microtubules (E7; red; second to the left), Acheron (anti-Acheron; green; second to the right), and composite (merge; far right) channels.
Figure 4b. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal From Figure 4a.
DAPI (blue), -tubulin (red), and Anti-Acheron (green) fluorescence intensity is shown on a graph in relation to cell surface area. While the numerous nuclei of the multi-nucleated myotube may be observed as independent DAPI fluorescence peaks, the intensity of the acheron (green) and -tubulin (red) fluorescence significantly overlaps.
We used human U2-OS osteosarcoma cells to investigate the idea that Acheron attaches to microtubules in non-muscle cells. A 15-year-old girl's moderately differentiated sarcoma of the tibia gave rise to the bone cancer cell line known as U2-OS (Rousseau, 2010). These cells' huge size and flat form make them often used in fluorescence microscopy (Rousseau, 2010). High resolution imaging and high accuracy colocalization analyses are made possible by these properties.
Additionally, U2-OS cells are a useful tool for expanding our findings since they endogenously express Acheron (Human Protein Atlas). The same procedures used for C2C12 myoblasts were used to prepare the samples for immunofluorescence, and they were then probed for Acheron (green), DNA (blue), and -tubulin (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a 100X magnification (Figure 5a). In the case of this Figure, the scanning confocal super resolution microscope's resolution and some good timing enabled us to see a cell moving through mitosis in order to collect extra data in addition to testing our theory. As might be predicted, DAPI staining can be detected in the cell's nucleus in Figure 5a, but there are also several distinct foci visible. Condensed chromosomes that are connected to kinetochore microtubules and aligning to the metaphase plate may be seen in this picture thanks to DAPI labelling. On the chromosomes, tension is being applied by the microtubules (red). Microtubules in cycling cells that are in metaphase and anaphase commit themselves to the process of cell division (Oriola et al.,2018). Acheron (green) is predominantly visible colocalizing with the cell's microtubules (yellow) (Figure 5a). The information from this picture, when combined, not only confirms our theory that Acheron attaches to microtubules in many mammalian lineages, but also shows that Acheron/microtubule interaction is maintained even during cell division phases when microtubules are used in different ways, such metaphase (Figure 5a).
We can visually see our colocalization in relation to the cell's size (in microns) by graphic analysis of the picture, and we can also determine the relative expression levels of our examined proteins and structures (Figure 5b). The blue line in this graph is DAPI staining, whereas the green and red lines, respectively, indicate Acheron and -tubulin. Acheron fluorescence can be detected nearly exactly matching the -tubulin fluorescence pattern, similar to Figures 3b, d, and 4b, whereas DAPI signal can be discretely seen. These results may be utilised to show that our yellow colocalization signalling really reflects colocalization rather than being an artefact of two signals layered together.
Figure 5a. Acheron’s Microtubule Localization is Preserved Between Different Mammalian Cell Lines.
A 100X confocal z-stack of a single labelled cycling U2-OS osteosarcoma cell's nuclei (DAPI; blue; far left), microtubules (E7; red; second from the left), Acheron (anti-Acheron; green; second from the right), and composite (merge; far right) channels. While chromosomal alignment to the metaphase plate can be identified using the DAPI signal, a significant percentage of the cell's Acheron can be seen colocalizing with microtubules in the yellow portions of the picture.
Figure 5b. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal From Figure 5a.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and Anti-Acheron (green) on the cell. While DAPI (blue) peaks can be detected in their own distinctive regions of the cell, Anti-Acheron (green) fluorescence intensity can be seen following the same distal trajectory as -tubulin (red).
We postulated that depolymerizing microtubules would release Acheron into the cytoplasm, allowing us to better assess if Acheron physically attaches to microtubules in mammalian cells. In order to dismantle microtubule connections and promote microtubule depolymerization, we used the anti-cancer drug Nocodazole to test this theory (Verstraelen et al., 2017). After giving Nocodazole to the cells, it was checked to see whether Acheron had localised to the microtubules.
I carried out a dose/response study to assess microtubule depolymerization using immunofluorescence and apoptosis in order to establish the best Nocodazole dosage (phase contrast imaging of membrane blebbing). These research' findings enabled us to identify the ideal Nocodazole treatment parameters that efficiently depolymerized microtubules while preventing cell death. Phase imaging allows for the observation of membrane blebbing and vesicle production at dosages of 750 nM for one hour (Figure 6a). Because dosages over this range would cause cell death, they were immediately disregarded from microtubule analysis. Nocodazole was diluted below 750 nM for an hour before cells were stained for microtubules (E7; magenta) and nuclei (DAPI; cyan), which were then photographed at 60X. (Figure 6b). Until 400 nM for 1 hour, nocodazole treatment failed to depolymerize microtubules in C2C12 myoblasts. Microtubules seem to have totally depolymerized at this time (Figure 6b). Together, the results of these investigations show that 400–750 nM for an hour is the optimal concentration and duration for Nocodazole-mediated microtubule depolymerization in cycling C2C12 myoblasts.
After that, 500 nM Nocodazole was applied to C2C12 cells for 1 hour. The cells were then fixed on poly-L-lysine-treated coverslips and probed for Acheron (green), DNA (blue), and -tubulin (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a 100X magnification (Figure 6c). Microtubule pieces may be observed in red in this illustration, which shows that the microtubule has completely depolymerized. However, in regions of yellow, Acheron (green) may still be observed localising to -tubulin (red) (Figure 6c). Perinuclear regions of the cell exhibit a buildup of Acheron and -tubulin, but the lack of membrane blebbing and vesicle formation indicates that the 500nM Nocodazole treatment did not cause the cell to undergo apoptosis (Figure 6c).
This data is visually displayed in Figure 6d. Acheron (green) and -tubulin (red) peaking may be detected in regions close to the DAPI (blue) signal, which indicates perinuclear accumulation.
Additionally, Acheron expression may be shown to follow a path similar to that of -tubulin, with decreased expression of Acheron in the cell's periphery (Figure 6d). These results confirm our earlier research, which suggests that Acheron binds to individual -tubulin monomers as well as microtubules (Figure 6c, Figure 6d).
Figure 6a. Phase Contrast Images of Nocodazole Treated C2C12 Myoblasts.
Cycling mononucleated C2C12 myoblasts' phase z-projections in response to various Nocodazole doses. Arrows point to regions that exhibit signs of apoptosis such vesicle production and membrane blebbing. imaged 60 times.
Figure 6b. Immunofluorescent Staining of Nocodazole Treated C2C12 Myoblasts.
Composite z-projections of microtubules (E7; magenta) and nuclei (DAPI; cyan) in cycling mononucleated C2C12 myoblasts in response to different Nocodazole doses. imaged 60 times.
Figure 6c. Acheron localizes to Individual β-tubulin Monomers in Nocodazole Treated C2C12 Myoblasts
A confocal z-stack of a single stained cycling mononucleated C2C12 myoblast treated with 500 nM Nocodazole for an hour, imaged at 100X, showing the nucleus (DAPI; blue; far left), microtubules (E7; red; second to the left), Acheron (anti-Acheron; green; second to the right), and composite (merge; far right) channels. Even though the bulk of the Acheron signal is peri-nuclear in this image, it is still possible to observe Acheron colocalizing with microtubules.
Figure 6d. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal From figure 6c.
DAPI (blue), -tubulin (red), and Anti-Acheron (green) fluorescence intensity is shown on a graph in relation to cell surface area. Despite microtubule depolymerization, DAPI signal can only be observed in the nucleus whereas Acheron can be seen in the same regions as depolymerized tubulin monomers.
BAD, HHARI and CASK Localization in C2C12 Myoblasts:
Acheron is essential to PCD, myogenesis, and pathogenesis processes. The BH3-only protein BAD, the E3 ubiquitin ligase HHARI, and the calcium/calmodulin-dependent serine protein kinase, CASK, are thought to interact with Acheron. Therefore, knowing how these chemicals are localised inside the cell in relation to Acheron and figuring out if these molecules are directly complexed with Acheron would be crucial knowledge about Acheron's function in these cellular processes. C2C12 myoblasts were plated on poly-L-lysine coverslips and immunostained for BAD, HHARI, and CASK in relation to microtubules in order to test the hypothesis that Acheron co-localizes with known binding partners (BAD, HHARI, and CASK) at the cell's microtubules. As stated before orange/yellow signals for the other photos may be used to estimate regions of colocalization between BAD, HHARI, CASK (green), and -tubulin (red).
In C2C12 myoblasts, co-IP of Acheron revealed a physical interaction with the BH3-only protein BAD (Sheel et al., 2020). I wanted to find out whether BAD could be discovered localising to microtubules in C2C12 myoblasts given our earlier discoveries that Acheron attaches to microtubules and that BAD could be found binding to Acheron. Stains for DNA (blue), BAD (green), and -tubulin were applied to myoblasts (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a 100X magnification (Figure 7a). The absence of yellow regions in the final composite picture suggests that there is no detectable colocalization. Instead of the filamentous pattern that is typical of Acheron, BAD (green) may be observed following a punctate pattern (Figures 3a; 3c; 4a; and 5a).
The fluorescence intensities of each colour channel are shown graphically to support our conclusion that BAD does not bind to microtubules in C2C12 myoblasts (Figure 7b). Here, the BAD (green) signal exhibits a fluorescence pattern distinct from that of -tubulin (red) and DAPI (blue). Similar to our previous expression of mitotracker, peaks of BAD expression may be found in perinuclear regions of the cell (Figures 1a-c). These findings do not, taken together, support the idea that BAD localises to microtubules in C2C12 myoblasts.
7a. Super Resolution Subcellular Localization of BAD Relative to Microtubules in C2C12 Myoblasts.
A 100X confocal z-stack of a single labelled cycling C2C12 myoblast's nuclei (DAPI; blue; far left), microtubules (-tubulin; red; second from the left), BAD (anti-BAD; green; second from the right), and composite (merge; far right) channels.
Figure 7b. Colocalization analysis of DAPI, BAD and β-tubulin Fluorescent Signal From Figure 7a.
DAPI (blue), -tubulin (red), and Anti-BAD (green) fluorescence intensity is shown on a graph in relation to cell surface area. While partially overlapping with the DAPI signal, the anti-BAD (green) fluorescence intensity can be observed travelling in a different distal direction from the -tubulin (red).
Acheron and Human Homolog of Ariadne-1 (HHARI), a ubiquitin ligase, were shown to be bound by Co-IP and Yeast 2-Hybrid screening (Wang Z, 2003). This information, together with the knowledge that Acheron attaches to microtubules in mammalian cells, prompted me to investigate the possibility that HHARI may also be connected to microtubules in mammalian cells.
DNA, HHARI, and -tubulin were all labelled on C2C12 cells in blue, green, and yellow (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a 100X magnification (Figure 8a). Similar to Figure 7a, there is no discernible area of yellow staining, indicating that colocalization is not present. Instead, HHARI is shown to collect in certain perinuclear regions of the cell. It may be assumed that HHARI is localising to the golgi apparatus, with some extra signal in the nucleoplasm, rather than being at the cell's centrosome since this region does not colocalize with microtubules (Figure 8a).
Figure 8b illustrates this in a visual way. The HHARI fluorescent signal is shown in green in this figure, whereas the -tubulin and DAPI signals are shown in red and blue, respectively. Here, HHARI seems to express independently of -tubulin, accumulating at one end of the nucleus and exhibiting extra expression inside the cell nucleus (Figure 8b).
The conclusion that HHARI attaches to microtubules in C2C12 myoblasts is not supported by our results.
8a. Super Resolution Subcellular Localization of HHARI Relative to Microtubules in C2C12 Myoblasts
A 100X confocal z-stack of a single labelled cycling C2C12 myoblast's nuclei (DAPI; blue; far left), microtubules (-tubulin; red; second from the left), HHARI (anti-HHARI; green; second from the right), and composite (merge; far right) channels.
Figure 8b. Colocalization analysis of DAPI, HHARI and β-tubulin Fluorescent Signal From Figure 8a.
DAPI (blue), -tubulin (red), and Anti-HHARI (green) fluorescence intensity is shown on a graph in relation to cell surface area. In this image, HHARI fluorescence is seen.
There are significant quantities outside the nucleus and minimal amounts within. The signals here do not match the fluorescence of -tubulin.
Acheron and mouse embryonic cDNA were subjected to yeast 2-hybrid screening, which demonstrated CASK interacts to the C-terminus of Acheron through CASK's CaM K II-like domain (Wang et al., Unpublished).
It is generally known that CASK is localised to the cytoplasm and nucleus of the cell. We hypothesised that some of CASK may localise to regions of known Acheron localisation in mammalian cells as a result of our discovery that CASK binds to Acheron, a protein that is localised to microtubules.
C2C12 myoblasts were stained for DNA (blue), CASK (green), and -tubulin to test the theory that CASK localises to microtubules of C2C12 cells (red). Then, using a Nikon A1R-SIMe resonant scanning confocal super resolution microscope, slides were scanned at a 100X magnification (Figure 9a). Similar to Figures 7a and 8a, this picture has no yellow coloration at all, suggesting that colocalization is not present. In accordance with data reported in the literature (Human Protein Atlas), CASK localises to the cytoplasm and nucleus in this figure (Figure 9a). Figure 9b makes this extremely clear. With the exception of a minor spike in CASK signal that overlaps with DAPI, which indicates that some CASK localises to the nucleus, CASK (green), tubulin (red), and DAPI (blue) signal seem to occupy separate sites inside the cell in the image (Figure 9b). These findings corroborate the idea that CASK localises to the nucleus, a region that is often linked to Acheron localization.
9a. Super Resolution Subcellular Localization of CASK Relative to Microtubules in C2C12 Myoblasts
A 100X confocal z-stack picture showing the cycling C2C12 Myoblasts' nuclei (DAPI; blue; far left), microtubules (E7; red; second from the left), CASK (anti-CASK; green; second from the right), and composite (merge; far right) channels.
Figure 9b. Colocalization analysis of DAPI, CASK and β-tubulin Fluorescent Signal From Figure 9a.
DAPI (blue), -tubulin (red), and anti-CASK (green) fluorescence intensity is shown on a graph in relation to cell surface area. Anti-CASK (green) fluorescence intensity can be observed in this figure travelling in a different distal route from -tubulin (red).
Anti-CASK expression may be detected accumulating in the nucleus close to the DAPI signal.
Nuclear export signals (NES) and functional nuclear localization signals (NLS) are features of the dynamic protein acheron (Valavanis et al., 2009; Shao et al., 2012). According to preliminary research, Acheron localises to the nucleus as well as microtubules (Fig. 1c). Growing and waning levels of growth factors, respectively, may cause Acheron to translocate into and out of the nucleus (Glenn et al., unpublished). Although CASK's cytoplasmic localization in the cytoplasm and nucleus of the cell has been well established (Gardener et al., 2006), CASK lacks a nuclear localization signal, hence the mechanism that promotes translocation is not entirely evident. According to earlier research, CASK is nuclear translocated as a consequence of ectopic expression of T-box transcription factor T-brain-1 (Tbr-1), which is situated in the cytoskeletal structure (Bredt et al., 2000). We wondered whether Acheron served as a shuttle protein for CASK entering the nucleus in light of these results and the knowledge that it binds to CASK and localises to microtubules.
We used two distinct lines of C2C12 cells producing ectopic Acheron to test the idea that CASK translocates to the nucleus when Acheron translocation is stimulated (Shao et al., 2012). A plasmid encoding the expression of full-length, HA-tagged Acheron, driven by a pCMV-Neo retroviral promoter, was transfected into the first line. A mutant Acheron that lacks the nuclear export signal was transfected into the second line (Shao et al., 2012). We can see the cells in a time-independent manner as a result of nuclear Acheron being locked in the nucleus.
Both lines were plated on coverslips coated with poly-L-lysine and developed in conventional C2C12 growth media. Cells were fixed when Acheron was lured into the nucleus by increasing growth factor concentrations as previously mentioned after they had attained 70% confluency. Then, cells were photographed at 100X while labelled for Acheron (green), -tubulin (red), and DAPI (blue) (Figure).
10a). For this experiment, the cell lines C2C12 + HA + Acheron and C2C12 + HA + Acheron -NES were used, and control pictures of both cell lines are shown in additional Figures 2a and 3a. Acheron is seen in these photos confined to the cell's microtubules. Acheron doesn't start to collect around the nucleus in NES cells until serum levels are high.
Figure 10a shows cells cultivated in conventional 10% FBS in the top half and cells cultured in 15% FBS with nonfunctional NES in the bottom half. In line with our earlier photos, acheron (green) can be shown confined to microtubules (red). The bright yellow colour in the composite picture on the right serves as evidence of this (Figure 10a). However, Acheron (green) got stuck in the nucleus (blue) and had reduced levels of expression linked to microtubules (red) when -NES cells were incubated in 15% FBS (Figure 10a).
Figures 10b and 10c show this in considerably more depth. The 10% FBS sample's fluorescence intensity are shown visually in Figure 10b. Following the expression of -tubulin (red), the Acheron fluorescence intensity (green) may be detected in this image. Low amounts of Acheron staining may be found in the nucleus close to the DAPI (blue) signal (Figure 10b).
Figure 10c, on the other hand, displays the fluorescence intensity of the 15% -NES sample. In this picture, Following the -tubulin's (red) fluorescence intensity, although to a lesser degree, is the expression of acheron (green). Instead, near the cell nucleus, sizable peaks of the Acheron (green) signal can be observed aggregating with the DAPI (blue) signal. Together, the findings show that Acheron may be pushed into the cell's nucleus in response to rising growth factor concentrations.
Figure 10a. Acheron Nuclear Translocation in Response to Increased Growth Factors in C2C12 Myoblasts.
A 100X confocal z-stack of a single labelled modified C2C12 cell showing the nuclei (DAPI; blue; far left), microtubules (E7; red; second from the left), Acheron (anti-Acheron; green; second from the right), and composite (merge; far right) channels.
Figure 10b. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal in C2C12 Myoblasts in 10% FBS.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and anti-Acheron (green) in 10% FBS samples. In this figure, the expression location for the -tubulin (red) signal matches the anti-Acheron (green) fluorescence intensity. DAPI (blue) signal is close to low levels of Achron (green) in the nucleus.
Figure 10c. Colocalization analysis of DAPI, Acheron and β-tubulin Fluorescent Signal in C2C12 Myoblasts in 15% FBS.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and anti-Acheron (green) in samples of 15% FBS. Unlike Picture 10b, which shows Acheron (green) aggregating around DAPI (blue) signal in the cell nucleus, this figure shows anti-Acheron (green) fluorescence intensity matching expression localisation for -tubulin (red) signal.
The experiment was repeated with the exception that slides were probed for CASK (green), -tubulin (red), and DNA (blue) in order to further investigate the idea that CASK translocates to the nucleus of mammalian cells when Acheron translocation is stimulated (Figure 11a).
The panel of photographs that results is structured similarly to Figure 10a. Cells that were not exposed to higher growth factor concentrations can be seen in the top half of the picture, whereas NES cells that were incubated in higher serum concentrations can be seen in the bottom half.
CASK (green), which is distinct from microtubules, is confined to the cytoplasm of the cell in the 10% FBS cells (red). This is consistent with the findings in Figure 9a. Different behaviour of CASK (green) is seen in the 15% FBS-NES cells. Contrary to earlier, there is a large rise in the expression of CASK (green) in these cell lines, and the green signal is far more common than its 10% FBS counterpart. Some of the signal localises to microtubules (red), as shown by the yellow colouring, with the majority of the signal also being visible in the nucleus (blue) (Figure 11a). In Figures 11b and 11c, the localisation of CASK in both samples is more clearly shown visually.
Figure 11b depicts the perinuclear space, where CASK fluorescence intensities (green) can be observed accumulating surrounding the DAPI (blue) signal. Additionally, the CASK (green) signal exhibits a fluorescence intensity pattern distinct from the -tubulin (red) signal (Figure 11b).
Figure 11c depicts the accumulation of CASK fluorescent signal (green) in the nucleus near DAPI (blue) signal to a larger degree than Figure 11b. Comparatively, this shows that CASK (green) successfully moved to the cell's nucleus in response to rising growth stimuli. The CASK (green) fluorescent signal can be observed closely following the -tubulin (red) signal in 15% FBS samples, in sharp contrast to our earlier CASK findings, demonstrating localization to microtubules (Figure 11c).
Figure 11a. CASK Nuclear Translocation in Response to Increased Growth Factors in C2C12 Myoblasts.
A 100X confocal z-stack of a single stained modified C2C12 cell showing the nuclei (DAPI; blue; far left), microtubules (E7; red; second from the left), CASK (anti-CASK; green; second from the right), and composite (merge; far right) channels.
.png)
Figure 11b. Colocalization analysis of DAPI, CASK and β-tubulin Fluorescent Signal in C2C12 Myoblasts in 10% FBS.
An illustration showing the relative fluorescence intensities of DAPI (blue), -tubulin (red), and Anti-CASK (green) in 10% FBS samples. In this image, anti-CASK (green) fluorescence can be seen adhering to -tubulin (red) along the fluorescent signal intensity channel and accumulating towards the DAPI (blue) signal in the perinuclear region.
Figure 11c. Colocalization analysis of DAPI, CASK and β-tubulin Fluorescent Signal in C2C12 Myoblasts in 15% FBS.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and Anti-CASK (green) in samples of 15% FBS. As compared to its 10% FBS counterpart, the anti-CASK (green) fluorescence intensity in this figure can be observed localising to the DAPI (blue) signal in higher amounts. In striking contrast to Figure 11b, the intensity of the CASK (green) fluorescence signal can be observed closely following the expression of -tubulin (red) as well.
Discussion:
The trials mentioned above have two different investigations as their goals. In the first investigation, I aimed to identify Acheron's subcellular location. I investigated the theories that Acheron localises to mitochondria, binds to actin, and binds to microtubules in mammalian cells in order to achieve this objective. I used developed C2C12 myotubes, U2-OS osteosarcoma cells, and undifferentiated C2C12 myoblasts. The idea that Acheron localization was taking place directly via interactions with tubulin was subsequently put to the test.
In the second experiment, I investigated the possibility that Acheron colocalizes with a set of known binding partners, including BAD, Human Homology of Ariadne-1 (HHARI), and Calcium/Calmodulin-dependent Serine Protein Kinase (CASK). I further explored the idea that Acheron may aid in the movement of CASK into mammalian cells' nuclei.
Overall, these research' findings indicate that Acheron binds to microtubules in several cell types and that this localisation is directly controlled by Acheron—tubulin binding. These research' findings, however, did not corroborate the idea that Acheron and BAD and Acheron and HHARI colocalize at the cell's microtubules. The results of this investigation did support the idea that Acheron could make it easier for CASK to enter mammalian cells' nuclei.
Figures 1-6 show the results of the first investigation. Acheron was mostly visible in the nucleus and followed a reticulated pattern in the cytoplasm in Figure 1a. However, the cytoplasmic signal had a particular reticulated pattern (Figure 1a). Graphically, the Acheron signal can be shown occupying area that is essentially independent of the Mitotracker fluorescence, despite the Acheron and Mitotracker signals' proximity to the nucleus (45 um and 90 um). Although not with the maxima of the DAPI (blue) signal detected about 55um and 75um, acheron fluorescence did seem to overlap with the DAPI signal in the nucleus. Figure 1a shows these peaks as the brighter blue patches that may be densely packed heterochromatin in the cell's nucleus (Linhoff et al., 2015). Although further direct testing is needed, the separation of Acheron fluorescence and heterochromatin supports the theories that, although being present in the nucleus, Acheron does not attach to the DNA directly. Acheron signal in the cytoplasm seen in Figure 1 is not diffuse, but rather follows a particular cytoskeletal structure, as can be seen by closer inspection. This prompted us to postulate that Acheron binds to a component of the cytoskeleton like actin or tubulin. Nuclear Acheron looks diffuse rather than following a recognisable pattern, in contrast to its cytoplasmic staining. It's interesting to note that nuclear and cytoskeletal signals are not necessary for perinuclear Acheron to seem to follow its own distinct pattern. A distinct virtually horizontal pattern can be seen projecting out of the nucleus into the cytoplasm, whereas Acheron can be seen around the nuclear membrane in Figure 1c's improved resolution. This prompts us to speculate that Acheron may be localising to ribosomes and the cell's rough endoplasmic reticulum, respectively.
Aggregates of Acheron were seen near the cell's perimeter, correlating with the results provided by Dermit et al. (2020). (Figure 1b). As would be predicted for lamellipodia, these regions of increased Acheron signal do not localise to the leading edge of the cell. Instead, these regions of increased Acheron signal localise to filopodia-like protrusions that protrude from the cell. This lends credence to recent research by Dermit et al. (2020) showing that Acheron is abundant at actin-rich cell protrusions. When combined, the findings from Figures 1a, 1b, and 1c lend credence to the idea that Acheron attaches to a cytoskeletal component there. Additionally, the data collected for this research gives us better resolution and details on Acheron's localization both inside the nucleus and in its enriched regions at the cell's filopodia.
But examination of the Acheron staining in this cell confirms many of the findings from Figures 1a, b, and c. A pattern seen in the nucleus of acheron is expressed more strongly in the perinuclear region. Acheron expression exhibits a reticulated pattern in the cytoplasm, according to a closer inspection (Figures 1a and 1c). As previously, brighter regions of enhanced Acheron signal are seen at the ends of actin filaments. This bolsters the claims that: a) the Acheron staining zones are real and consistent with the results provided by Dermit et al. (2020); and b) the enhanced areas may be detected at the cell's filopodia rather than merely at cellular protrusions. Acheron does not localise to actin filaments, despite the fact that filopodia are actin-rich structures, as shown by the information above (Figures 2a and 2b). We examined cells for microtubules, a distinctive cytoskeletal component linked to filopodia, in an effort to solve this puzzle. Figures 3a and 3c show the results of our Acheron, DNA, and microtubule probes on cycling C2C12 myoblasts. In contrast to Figures 1 and 2, the cytoplasmic Acheron and microtubule fluorescence signals are colocalized in these photos, to the best of their ability. Fluorescence in the yellow colour indicates this (Figures 3a, 3c). The idea that Acheron localises to microtubules in mammalian cell lines is supported by this.
We differentiated C2C12 myoblasts into myotubes and stained them for Acheron, DNA, and microtubule filaments to test the theory that Acheron-microtubule localization is maintained with throughout development (Figure 4a). In the ensuing image, we discovered that Acheron expression had been significantly elevated and that, in contrast to the predominant nuclear staining observed in cycling cells, the signal was mostly cytoplasmic and linked to the cytoskeleton. Figure 4a shows this visually, and Figure 4b shows it graphically. When DAPI expression increases, Acheron expression dips, indicating that in differentiated myotubes, Acheron has a preference for the cytoplasm rather than the nucleus (Figure 4b). This implies that in mouse myoblasts and myotubes, acheron location is developmentally controlled. This leads us to hypothesise that Acheron is limited to the cytoplasm after differentiation and has functions in the nucleus and cytoplasm of cycling cells. (See Figure 4a.)
We immunostained cycling U2OS human osteosarcoma cells for Acheron, DNA, and microtubules in order to further our investigations and ascertain if Acheron binding to microtubules is exclusive to C2C12 cells or a more widespread phenomena (Figure 5a). In U2OS cells, Acheron binds to microtubules, and this was verified. The yellow fluorescence in the composite channel and the overlap between the graphed Acheron (green) and -tubulin (red) fluorescence intensities serve as examples of this (Figure 5a and 5b). We also see nuclear Acheron staining (green), which appears to be excluded from the DNA DAPI (blue) signal, which is consistent with the findings from C2C12 cells (Figure 5b). We had the good fortune to photograph a cell during the metaphase of mitosis for this figure. As they align to the metaphase plate, the condensed chromosomes may be observed connected to kinetochore microtubules. Microtubules and Acheron co-localize, but not chromosomes (Figure 5a and 5b). Considering all of the information, it is suggested that Acheron attaches to microtubules and that this Acheron-microtubule interaction is conserved in mammalian species. Additionally, it supports the idea that nuclear Acheron does not directly attach to DNA.
Finally, cycling C2C12 myoblasts were treated with Nocodazole and probed for Acheron, DNA, and microtubules in order to test the idea that Acheron-microtubule localization is preserved when susceptible to microtubule depolymerization (Figure 6c). In this case, 500 nM of nocodazole for an hour was shown to be the best dosage. It was discovered that although this dose completely depolymerizes microtubules, it does not instantly cause apoptosis (Figures 6a and 6b). Surprisingly, Acheron was discovered to maintain microtubule binding even after depolarization, as seen by yellow fluorescence in the composite channel. Although the acheron signal is obviously diffuse, it is colocalized with -tubulin monomers as seen by the yellow fluorescence and signal overlap that can be detected (Figure 6c and 6d). This offers fresh understanding of Acheron's microtubule localisation. In light of the aforementioned information, the results of this test are consistent with the idea that Acheron localises to microtubules in mammalian cells and that this localization is unique to the monomer of the microtubule, -tubulin (Figure 6c and 6d).
Acheron's La theme lends more weight to this assertion. A functional nuclear localization signal (NLS), a nuclear export signal (NES), a Suz-C domain (subcellular localization and mRNA substrate recognition), a well-conserved RNA recognition motif (RRM), and a functional, winged helix-turn-helix structured RNA binding, LA motif (LAM) are all present in Acheron's structure (Stefanovic et al., 2014; Martino et al., 2015; Bousquet-Antonelli et al., 2009; Dong et al., 2004; Valavanis et al., 2007; Stavraka et al., 2015). Individual alpha and beta tubulin monomers interact with winged-helix protein domains through its carboxy-terminus, according to X-ray crystallography and crosslinking/mass spectrometry (MS) findings (Abad et al., 2014).
The localization of Acheron in the nucleus, perinuclear, and filopodia, as well as other aspects of Acheron localization, are all revealed by Acheron staining under diverse experimental settings. Acheron's perinuclear expression is further clarified by the enhanced yellow fluorescence in the nucleus. While this increase in Acheron expression could indicate that the protein localises to the cell's Golgi apparatus or rough endoplasmic reticulum, the presence of yellow colocalization with microtubules tells us that this localization is more likely to the centrosome, one of the main microtubule organising centres of the cell. Filopodia have a lot of actin (Mattila and Lappalainen, 2008). We discovered that Acheron is concentrated at the filopodia but does not bind with phalloidin-stained structures inside the cell (Figure 2). (Figure 2b). The role of microtubules in cell proliferation provides the best explanation for this occurrence. Microtubules and actin filaments compete with one another to control cell mobility inside growth cone-like structures during cell proliferation and migration (Khan and Baas, 2016). Growth cones' main structures are filopodia (Davenport et al., 1993). Acheron may then be restricted to the microtubules in these formations.
The theory that microtubules may directly engage in directed cell motility via filopodia merging and modulation of filopodia density is supported by recent findings as well (Schober et al., 2007).
As a result, one hypothesis is that Acheron may likewise be abundant and localised at the microtubules that regulate the movements of filopodia.
The nucleus exhibits relative levels of the Acheron signal in practically all experimental circumstances. However, the peaks of brighter DAPI expression never correspond with the graphed expression intensities of nuclear Acheron (green) (blue). The information presented in this study suggests that, although Acheron localises to the nucleus, it does not bind directly to DNA and, as a result, does not become enriched at bundles of heterochromatin or condensed chromosomes, contrary to previous studies that have shown pericentric heterochromatin is easily detectable as large DAPI-dense foci (Imai et al., 2017). This sheds fresh light on the mechanism controlling Acheron's RNA binding protein function.
Numerous mRNA molecules reside in the cell's nucleoli. Numerous RNA-binding proteins may also be discovered localised in nucleoli as a result (Nazer et al., 2011). Recent information, however, has highlighted the significance of heterochromatin localization to the cell's nucleoli's periphery. Super resolution microscopy's increased resolution reveals that Acheron does not localise to DNA, and consequently heterochromatin itself, and that Acheron is not present in or around nucleoli (Guetg and Santoro, 2012). This information, along with the fact that Acheron has been shown to play a role in the biogenesis of ribosomes (Dermit et al., 2020), a process that starts in the nucleolus and ends in the cytoplasm of eukaryotic cells, leads us to conclude that Acheron's role in ribosome biogenesis is independent of nucleoli localization and must take place in cytoplasm (Pelletier et al., 2018).
Ribosome biogenesis begins in the nucleolus, where pre-ribosome assembly (pre-40s, pre-60s) takes place. Pre-ribosomes that have been synthesised then go from the nucleoplasm into the cytoplasm, where they develop into the ribosomal 40S and 60S subunits that make up the ribosome (Thomson et al., 2013). When combined, the results of these investigations imply that Acheron controls ribosome biogenesis by using ribosomal subunits. Future research is needed to look at this, however.
In conclusion, the research's studies show that Acheron is abundant in the growth cones of the filopodia and localises to microtubules in mammalian cells, particularly to individual -tubulin monomers. Finally, the evidence points to the possibility that nuclear Acheron may not directly attach to DNA; however, further research is needed to confirm this.
In C2C12 myoblasts, co-IP of Acheron revealed a physical interaction with the BH3-only protein BAD (Sheel et al., 2020). I wanted to find out whether BAD could also be seen localising to microtubules in C2C12 myoblasts given our previous discoveries that Acheron binds to both microtubules and BAD. The findings of this research indicate that BAD does not reside on microtubules (Figure 7). Super resolution microscopy does not support the idea that Acheron and BAD colocalize at the cell's microtubules together, despite co-IP results showing a physical contact between Acheron and the BH3-only protein BAD in C2C12 myoblasts. Post-translational changes of BAD are one theory that might be used.
Five serines in the structure of BAD may be phosphorylated by various kinases (Burlacu et al., 2003). Ser112, Ser128, Ser136, Ser155, and Ser179 are some of these locations. BAD Ser128 may be detected localised to the mitochondria, according to immunofluorescent examination (Yang et al., 2008). BAD, however, is phosphorylated at Ser155, which causes it to go into the cytoplasm and prevents it from attaching to Bcl-2 (Datta et al., 2000). Future studies looking at BAD/Acheron co-localization should keep an eye on where BAD is located in relation to its level of phosphorylation. Phosphorylation often causes conformational changes, which expose residues for several possible binding partners (Groban et al., 2006). We may expand our co-IP data by tracking the localisation of BAD in different phosphorylated states. It was discovered by Co-IP and yeast 2-Hybrid screening that Acheron interacts to the human homolog of Ariadne-1, a ubiquitin E3 ligase (HHARI). Cycling C2C12 myoblasts were immunostained for HHARI, tubulin, and DNA to test the theory that HHARI may be detected localised to microtubules in mammalian cells (Figure 8). HHARI did not localise to the microtubules, as was discovered. This information contradicts earlier results from Co-IP and yeast 2-Hybrid showing HHARI interacts to the microtubule-binding protein Acheron. The peri-nuclear signal from Acheron and HHARI might provide one reason for this disparity.
HHARI does not localise to microtubules, as we have shown. However, the region surrounding the nucleus is rich in both HHARI and Acheron (Figures 3b & 8b). Microtubule-organizing centres (MTOCs) like the centrosome are crucial for microtubule organisation. Microtubule organisation, nucleation, and attachment of microtubule minus ends occur at these locations (Conduit et al., 2015). According to recent research, the Golgi apparatus is another location where microtubules may form and become stable (Chabin-Brion et al., 2001).
As a result, polarised microtubules that originate from the Golgi may encourage cell asymmetry and the polarised transport of post-Golgi carriers necessary for cell migration (Vinogradova et al., 2012). Given that HHARI does not localise to microtubules, it may be assumed that HHARI is localising to the Golgi apparatus rather than centrosomal localization as with Acheron from the rise in perinuclear fluorescence (Figure 8).
The additional information reported in this thesis provides support for one possibility explaining the HHARI/Acheron binding seen using co-IP and Yeast-2-Hybrid. Acheron is more abundant near the cell's filopodia, likely in the growth cones of proliferating cells (Dermit et al., 2020). (Figures 1-6). Dynamic growth cone rotation in proliferating cells requires polarised invasion of microtubules into one side of the peripheral domain of the growth cone (Kahn et al., 2016). Given that the Golgi apparatus functions as an MTOC of polarised microtubules, it is possible that the localization of HHARI to the Golgi and the enrichment of Acheron in growth cones of proliferating cells are related. Analysis of Acheron enrichment at the filopodia in HHARI-deficient cells has to be done in more research.
Acheron and mouse embryonic cDNA were subjected to yeast 2-hybrid screening, which demonstrated CASK interacts to the C-terminus of Acheron through CASK's CaM K II-like domain (Wang et al., unpublished).
It is already well known that CASK localises to the cytoplasm and nucleus of the cell (Ojeh et al., 2008). Acheron localises to the cell's microtubules and nucleus, according to earlier research. I stained cycling C2C12 myoblasts for CASK (green), -tubulin (red), and DAPI to test the idea that CASK localises to the microtubules of mammalian cells (blue). According to the results of this study, CASK does not bind to microtubules in mammalian cells.
Acheron and CASK are revealed by colocalization studies, but they bind in yeast-2-hybrid experiments. The capacity of Acheron to move into and out of the cell's nucleus is one tenable explanation for this data difference.
A functional nuclear export signal (NES) and nuclear localization signal (NLS) are features of the dynamic protein acheron (Shao et al., 2012). Additionally, earlier research from the lab has shown that Acheron translocation may be initiated in response to both rising and decreasing growth factors, respectively (Glenn et al., unpublished). Although CASK is canonically located in the cytoplasm and nucleus of the cell, it lacks a nuclear localization signal (NLS) (Gardener et al., 2006). Because of this, it is unknown what causes its translocation. One hypothesis is that when Acheron shuttles CASK into the nucleus, a brief period of CASK-Acheron binding takes place.
In Figures 10 and 11, I investigated the claim that, in response to rising growth factors, CASK translocation to the nucleus is linked with Acheron translocation to the nucleus. Together, the findings from this research demonstrate that, in response to rising growth factors, CASK translocation to the nucleus is linked with Acheron translocation to the nucleus. Furthermore, the information shown in Figure 11a is consistent with the theory that CASK colocalizes with Acheron at the cell's microtubules during its translocation (Sonnen et al., 2012). (Figures 1-6).
In conclusion, our observations are consistent with the idea that Acheron is concentrated in the growth cones of the filopodia and localises to microtubules, particularly to individual -tubulin monomers. The nuclear acheron does not attach to DNA directly (future studies are required). On the microtubules of cycling mammalian cells, Acheron, BAD, and HHARI do not colocalize. In response to rising growth factors, CASK translocation to the nucleus is associated with Acheron translocation to the nucleus, and ultimately CASK colocalizes with Acheron at the cell's microtubules during its translocation.
Although noteworthy, these results highlight the need for further research to not only examine the processes governing the localization of Acheron, HHARI, BAD, and CASK in mammalian cells but also to validate the information provided above. To verify the visual information provided by fluorescence microscopy above, appropriate biochemical tests such as fractionation and Western blotting, co-IPs, FRET, and should all be carried out. Future research will examine the distribution of BAD and Acheron in different phosphorylated states in mammalian cell lines, the enrichment of Acheron at the filopodia of HHARI-deficient and wild-type mammalian cells, and the distribution of Acheron and CASK in different cellular compartments in response to increasing growth factors, among other things.
Supplemental Figures:
Supplemental Figure 1a. Super Resolution Subcellular Localization of Acheron Relative to Microtubules in C2C12 Myoblasts Without -NES Deletion.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and Anti-Acheron (green) in samples of 15% FBS.
Supplemental Figure 2a. Super Resolution Subcellular Localization of Acheron Relative to Microtubules in -NES C2C12 Myoblasts.
An illustration showing the relative fluorescence intensity of DAPI (blue), -tubulin (red), and Anti-Acheron (green) in samples of 15% FBS. In contrast to its 10% FBS counterpart, anti-CASK (green) fluorescence intensity can be observed localising to DAPI (blue) signal in this figure. In striking contrast to Figure 11b, the intensity of the CASK (green) fluorescence signal can be observed closely following the expression of -tubulin (red) as well.
Table 2a. Appendix of Key Figures and Single Slice Representations:
The file names, cell lines used, staining colour combinations, and single slice representative pictures of the important figures used in this dissertation are included in a supplementary table.
References
Abad, M. A., Medina, B., Santamaria, A., Zou, J., Plasberg-Hill, C., Madhumalar, A., Jayachandran, U., Redli, P. M., Rappsilber, J., Nigg, E. A., & Jeyaprakash, A. A. (2014). Structural basis for microtubule recognition by the human kinetochore Ska complex. Nature communications, 5, 2964. https://doi.org/10.1038/ncomms3964
Aguilera M., Oliveros M., Martinez-Padron M., Barbas J. A. and Ferrus A. (2000). Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of ring-finger proteins. Genetics 155, 1231-1244.
Beacham, D. A., Amatangelo, M. D., & Cukierman, E. (2006). Preparation of extracellular matrices produced by cultured and primary fibroblasts. Current protocols in cell biology, 33(1), 10-9.
Beaulaton, J., & Lockshin, R. A. (1977). Ultrastructural study of the normal degeneration of the intersegmental muscles of Antheraea polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference to cellular autophagy. Journal of morphology, 154(1), 39-57.
Bornens, M., & Azimzadeh, J. (2007). Origin and evolution of the centrosome. In Eukaryotic Membranes and Cytoskeleton (pp. 119-129). Springer, New York, NY.
Bratton, S. B., Walker, G., Srinivasula, S. M., Sun, X. M., Butterworth, M., Alnemri, E. S., & Cohen, G. M. (2001). Recruitment, activation and retention of caspases?9 and?3 by Apaf? 1 apoptosome and associated XIAP complexes. The EMBO journal, 20(5), 998-1009.
Burlacu A. (2003). Regulation of apoptosis by Bcl-2 family proteins. Journal of cellular and molecular medicine, 7(3), 249–257. https://doi.org/10.1111/j.1582-4934.2003.tb00225.
Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal.2012;24(6):1297–1305.
Cai L, Fritz D, Stefanovic L, Stefanovic B. (2010) Binding of LARP6 to the conserved 5’ stem loop regulates translation of mRNAs encoding type I collagen. J Mol Biol. 395:30926.
Chabin-Brion, K., Marceiller, J., Perez, F., Settegrana, C., Drechou, A., Durand, G., & Pous, C. (2001). The Golgi complex is a microtubule-organizing organelle. Molecular biology of the cell, 12(7), 2047-2060.
Conduit, P. T., Wainman, A., & Raff, J. W. (2015). Centrosome function and assembly in animal cells. Nature reviews Molecular cell biology, 16(10), 611-624.
Davenport, R. W., Dou, P., Rehder, V., & Kater, S. B. (1993). A sensory role for neuronal growth cone filopodia. Nature, 361(6414), 721-724.
Datta, S. R., Katsov, A., Hu, L., Petros, A., Fesik, S. W., Yaffe, M. B., & Greenberg, M. E. (2000). 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Molecular cell, 6(1), 41-51.
Delbridge, A. R., Grabow, S., Strasser, A., & Vaux, D. L. (2016). Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nature reviews Cancer, 16(2), 99.
Dermit, M., Dodel, M., Lee, F., Azman, M. S., Schwenzer, H., Jones, J. L., Blagden, S. P., Ule, J., & Mardakheh, F. K. (2020). Subcellular mRNA Localization Regulates Ribosome Biogenesis in Migrating Cells. Developmental cell, 55(3), 298–313.e10. https://doi.org/10.1016/j.devcel.2020.10.006
Dimitratos, S. D., Stathakis, D. G., Nelson, C. A., Woods, D. F., & Bryant, P. J. (1998). The Location of HumanCASKat Xp11. 4 Identifies This Gene as a Candidate for X-Linked Optic Atrophy. Genomics, 51(2), 308-309.
Dong, G., Chakshusmathi, G., Wolin, S. L., & Reinisch, K. M. (2004). Structure of the La motif: a winged helix domain mediates RNA binding via a conserved aromatic patch. The EMBO journal, 23(5), 1000–1007. https://doi.org/10.1038/sj.emboj.7600115
Dumontet, C., Durán, G. E., Steger, K. A., Murphy, G. L., Sussman, H. H., & Sikic, B. I. (1996).
Differential expression of tubulin isotypes during the cell cycle. Cell motility and the cytoskeleton, 35(1), 49-58.
Eckelman, B. P., Salvesen, G. S., & Scott, F. L. (2006). Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO reports, 7(10), 988-994.
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic pathology, 35(4), 495-516.
Eskes, R., Desagher, S., Antonsson, B., & Martinou, J. C. (2000). Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Molecular and cellular biology, 20(3), 929-935.
Gardner, K. L., Sanford, J. L., Mays, T. A., & Rafael?Fortney, J. A. (2006). CASK localizes to nuclei in developing skeletal muscle and motor neuron culture models and is agrin? independent. Journal of cellular physiology, 206(1), 196-202.
Ghosh, A. K. (2002). Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Experimental biology and medicine, 227(5), 301-314.
Goldstein, J. C., Waterhouse, N. J., Juin, P., Evan, G. I., & Green, D. R. (2000). The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature cell biology, 2(3), 156-162.
Green, D. R., & Llambi, F. (2015). Cell death signaling. Cold Spring Harbor perspectives in biology, 7(12), a006080.
Groban, E. S., Narayanan, A., & Jacobson, M. P. (2006). Conformational changes in protein loops and helices induced by post-translational phosphorylation. PLoS Comput Biol, 2(4), e32.
Guetg, C., & Santoro, R. (2012). Formation of nuclear heterochromatin: the nucleolar point of view. Epigenetics, 7(8), 811-814.
Gunning, P. W., Ghoshdastider, U., Whitaker, S., Popp, D., & Robinson, R. C. (2015). The evolution of compositionally and functionally distinct actin filaments. J Cell Sci, 128(11), 2009-2019.
Hau, H., Ogundele, O., Hibbert, A. H., Monfries, C., Exelby, K., Wood, N. J., Nevarez-Mejia, J., Carbajal, M. A., Fleck, R. A., Dermit, M., Mardakheh, F. K., Williams-Ward, V. C., Pipalia, T. G., Conte, M. R., & Hughes, S. M. (2020). Maternal Larp6 controls oocyte development, chorion formation and elevation. Development (Cambridge, England), 147(4), dev187385. https://doi.org/10.1242/dev.187385
Hsueh, Y. P. (2006). The role of the MAGUK protein CASK in neural development and synaptic function. Current medicinal chemistry, 13(16), 1915-1927.
Hutt, K. J. (2015). The role of BH3-only proteins in apoptosis within the ovary. Reproduction, 149(2), R81-R89.
Imai, R., Nozaki, T., Tani, T., Kaizu, K., Hibino, K., Ide, S., ... & Maeshima, K. (2017). Density imaging of heterochromatin in live cells using orientation-independent-DIC microscopy. Molecular biology of the cell, 28(23), 3349-3359.
Irmler, M., Thome, M., Hahne, M., Schneider, P., Hofmann, K., Steiner, V., ... & Rimoldi, D. (1997). Inhibition of death receptor signals by cellular FLIP. Nature, 388(6638), 190-195.
Jensen, E. (2014). Technical review: colocalization of antibodies using confocal microscopy. The Anatomical Record, 297(2), 183-187.
Jiang, Peng et al. “The Bad guy cooperates with good cop p53: Bad is transcriptionally up- regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis.” Molecular and cellular biology vol. 26,23 (2006): 9071-82. doi:10.1128/MCB.01025-06
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141(1):52-67. https://www.sciencedirect.com
Kahn, O. I., & Baas, P. W. (2016). Microtubules and growth cones: motors drive the turn. Trends in neurosciences, 39(7), 433-440.
Kim, H. E., Du, F., Fang, M., & Wang, X. (2005). Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proceedings of the National Academy of Sciences, 102(49), 17545-17550.
Linhoff, M. W., Garg, S. K., & Mandel, G. (2015). A high-resolution imaging approach to investigate chromatin architecture in complex tissues. Cell, 163(1), 246-255.
Lockshin RA, and Williams CM. (1965) Programmed Cell Death--I. Cytology Of Degeneration In The Intersegmental Muscles Of The Pernyi Silkmoth. J Insect Physiol. 1965 Feb;11:123-33.
Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D., & Darnell, J. (2000). Molecular cell biology 4th edition. National Center for Biotechnology Information, Bookshelf.
Malladi, S., Challa?Malladi, M., Fearnhead, H. O., & Bratton, S. B. (2009). The Apaf?1• procaspase?9 apoptosome complex functions as a proteolytic?based molecular timer. The EMBO journal, 28(13), 1916-1925.
Manojlovic, Z., & Stefanovic, B. (2012). A novel role of RNA helicase A in regulation of translation of type I collagen mRNAs. Rna, 18(2), 321-334.
Manojlovic, Z., Blackmon, J., & Stefanovic, B. (2013). Tacrolimus (FK506) prevents early stages of ethanol induced hepatic fibrosis by targeting LARP6 dependent mechanisms of collagen synthesis. PLoS One, 8(6).
Marin I. and Ferrus A. (2002) Comparative genomics of the RBR family, including the Parkinson's disease-related gene parkin and the genes of the ariadne subfamily. Mol Biol Evol 19, 2039-2050.
Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5(9):2736-2749. https://www.openaire.eu/search/publication?articleId=od 267::af0bdcc61c88b887 1b194060911a1dbf.
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114: 181–190, 2003. doi:10.1016/S0092-8674(03)00521-X.
Ming, X. Y., Fu, L., Zhang, L. Y., Qin, Y. R., Cao, T. T., Chan, K. W., ... & Guan, X. Y. (2016).
Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nature communications, 7(1), 1-14.
Miura, M., Zhu, H., Rotello, R., Hartwieg, E. A., & Yuan, J. (1993). Induction of apoptosis in fibroblasts by IL-1β-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell, 75(4), 653-660.
Moldoveanu, T., & Czabotar, P. E. (2020). BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harbor Perspectives in Biology, 12(4), a036319.
Moynihan T. P., Ardley H. C., Nuber U., Rose S. A., Jones P. F., Markham A. F., Scheffner M. and Robinson P. A. (1999) The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1. J Biol Chem 274, 30963-30968.
Muñoz-Pinedo, C., Guío-Carrión, A., Goldstein, J. C., Fitzgerald, P., Newmeyer, D. D., & Green, D. R. (2006). Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proceedings of the National Academy of Sciences, 103(31), 11573-11578.
Nagata S, Tanaka M. Programmed cell death and the immune system. Nature reviews.
Immunology. 2017;17(5):333-340. https://www.ncbi.nlm.nih.gov/pubmed/28163302. doi: 10.1038/nri.2016.153.
Názer, E., Verdún, R. E., & Sánchez, D. O. (2011). Nucleolar localization of RNA binding proteins induced by actinomycin D and heat shock in Trypanosoma cruzi. PLoS One, 6(5), e19920.
Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol.
2001;41:367–401.
Ojeh, N., Pekovic, V., Jahoda, C., & Määttä, A. (2008). The MAGUK-family protein CASK is targeted to nuclei of the basal epidermis and controls keratinocyte proliferation. Journal of cell science, 121(16), 2705-2717.
Oriola, D., Needleman, D. J., & Brugués, J. (2018). The physics of the metaphase spindle. Annual review of biophysics, 47, 655-673.
Pawley, J. (Ed.). (2006). Handbook of biological confocal microscopy (Vol. 236). Springer Science & Business Media.
Pelletier, J., Thomas, G., & Volarevi?, S. (2018). Ribosome biogenesis in cancer: new players and therapeutic avenues. Nature Reviews Cancer, 18(1), 51-63.
Peng, J., Ding, J., Tan, C., Baggenstoss, B., Zhang, Z., Lapolla, S. M., & Lin, J. (2009).
Oligomerization of membrane-bound Bcl-2 is involved in its pore formation induced by tBid. Apoptosis, 14(10), 1145-1153.
Reed JC. Dysregulation of apoptosis in cancer. Journal of Clinical Oncology. 1999;17(9). Rousseau, J., Lamoureux, F., Picarda, G., Gouin, F., Trichet, V., & Rédini, F. (2010). Animal
Models of Malignant Primary Bone Tumors and Novel Therapeutic Approaches. In Bone cancer (pp. 333-346). Academic Press.
Scaffidi, C., Kirchhoff, S., Krammer, P. H., & Peter, M. E. (1999). Apoptosis signaling in lymphocytes. Current opinion in immunology, 11(3), 277-285.
Schober, J. M., Komarova, Y. A., Chaga, O. Y., Akhmanova, A., & Borisy, G. G. (2007).
Microtubule-targeting-dependent reorganization of filopodia. Journal of cell science, 120(7), 1235-1244.
Shao, R., Scully, S. J., Jr, Yan, W., Bentley, B., Mueller, J., Brown, C., Bigelow, C., & Schwartz,
L. M. (2012). The novel lupus antigen related protein acheron enhances the development of human breast cancer. International journal of cancer, 130(3), 544–554. https://doi.org/10.1002/ijc.26015
Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes & cancer.
2011;2(12):1097-1105. https://www.ncbi.nlm.nih.gov/pubmed/22866201. doi: 10.1177/1947601911423031.
Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–6.
Sonnen, K. F., Schermelleh, L., Leonhardt, H., & Nigg, E. A. (2012). 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biology open, 1(10), 965-976.
Stefanovic, L., Longo, L., Zhang, Y., & Stefanovic, B. (2014). Characterization of binding of LARP6 to the 5’stem-loop of collagen mRNAs: Implications for synthesis of type I collagen. RNA biology, 11(11), 1386-1401.
Strawbridge, R. J., Dupuis, J., Prokopenko, I., Barker, A., Ahlqvist, E., Rybin, D., ... & Nica, A. (2011). Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes, 60(10), 2624-2634.
Sun, R., Chen, W., Zhao, X., Li, T., & Song, Q. (2011). Acheron regulates vascular endothelial proliferation and angiogenesis together with Id1 during wound healing. Cell biochemistry and function, 29(8), 636-640.
Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB (Oct 1988). "MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts". Science. 242 (4877): 405–511.
Tait, S. W., & Green, D. R. (2010). Mitochondria and cell death: outer membrane permeabilization and beyond. Nature reviews Molecular cell biology, 11(9), 621-632.
Thomson, E., Ferreira-Cerca, S., & Hurt, E. (2013). Eukaryotic ribosome biogenesis at a glance. Tsujimoto, Y. (1998). Role of Bcl?2 family proteins in apoptosis: apoptosomes or mitochondria?.
Genes to cells, 3(11), 697-707.
Verstraelen, P., Detrez, J. R., Verschuuren, M., Kuijlaars, J., Nuydens, R., Timmermans, J. P., & De Vos, W. H. (2017). Dysregulation of microtubule stability impairs morphofunctional connectivity in primary neuronal networks. Frontiers in cellular neuroscience, 11, 173.
Vinogradova, T., Paul, R., Grimaldi, A. D., Loncarek, J., Miller, P. M., Yampolsky, D., ... & Kaverina, I. (2012). Concerted effort of centrosomal and Golgi-derived microtubules is
required for proper Golgi complex assembly but not for maintenance. Molecular biology of the cell, 23(5), 820-833.
Vukmirovic, M., Manojlovic, Z., & Stefanovic, B. (2013). Serine-threonine kinase receptor- associated protein (STRAP) regulates translation of type I collagen mRNAs. Molecular and cellular biology, 33(19), 3893-3906.
Wajant, H. (2002). The Fas signaling pathway: more than a paradigm. Science, 296(5573), 1635- 1636.
Wang, C., Li, J., Ye, S., Zhang, Y., Li, P., Wang, L., & Wang, T. H. (2018). Oestrogen inhibits VEGF expression and angiogenesis in triple-negative breast cancer by activating GPER-
1. Journal of Cancer, 9(20), 3802.
Wang, H., & Stefanovic, B. (2014). Role of LARP6 and Nonmuscle Myosin in Partitioning of Collagen mRNAs to the ER Membrane. PLoS One, 9(10), e108870.
Wang Z. (2003) Acheron, a novel regulator of myoblast differentiation. PhD Thesis, University of Massachusetts at Amherst.
Weigand, J. E., Boeckel, J. N., Gellert, P., & Dimmeler, S. (2012). Hypoxia-induced alternative splicing in endothelial cells. PloS one, 7(8).
Weng, H., Kim, C., Valavanis, C., Wang, Z., & Schwartz, L. (2009). Acheron, an novel LA antigen family member, binds to CASK and forms a complex with Id transcription factors. Cellular and Molecular Biology Letters, 14(2), 273-287.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074- 7613(95)90057-8.
William D. Foulkes, M.B., B.S., Ian E. Smith, Jorge S. Reis-Filho. Triple-negative breast cancer.
. . doi: 10.1056/NEJMra1001389.
Yaffe, D., & Saxel, O. (1977). Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature, 270(5639), 725–727. https://doi.org/10.1038/270725a0
Yang, X., Luo, C., Cai, J., Pierce, W. M., & Tezel, G. (2008). Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Investigative ophthalmology & visual science, 49(6), 2483-2494.
Yuan, S., Yu, X., Topf, M., Ludtke, S. J., Wang, X., & Akey, C. W. (2010). Structure of an apoptosome-procaspase-9 CARD complex. Structure, 18(5), 571-583.
Yu, X., Acehan, D., Ménétret, J. F., Booth, C. R., Ludtke, S. J., Riedl, S. J., ... & Akey, C. W. (2005). A structure of the human apoptosome at 12.8 Å resolution provides insights into this cell death platform. Structure, 13(11), 1725-1735.
Zhang, Y., & Stefanovic, B. (2017). mTORC1 phosphorylates LARP6 to stimulate type I collagen expression. Scientific reports, 7(1), 1-15.
Zou, H., Henzel, W. J., Liu, X., Lutschg, A., & Wang, X. (1997). Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c–dependent activation of caspase-3. Cell, 90(3), 405-413.
Coursework
Gonocytes coursework Assignment Sample
Question
Task: Write a Cell Profile on Gonocytes which are found in male testis developed from Germ Cells. The key points are provided which are to be followed when writing each section and starting references have been provided.
The marking rubric for the assignment is also attached.
Further detailed instructions are provided below:
Introduction:
Cell type – Gonocytes
This section should be around 3 pages long including images.
• Provide an introductory paragraph summarising about Gonocytes.
• What is the function of Gonocytes? Is the function different in the embryo and adult?
3) Where are Gonocytes located?
Choose region in which Gonocytes are found and focus on that and mention where else it is found in the embryo?
4) What processes does it go through during embryogenesis (and later) to become the final, functional Gonocyte ? Outline how Gonocytes are developed fertilisation onwards? (eg: Process of how germ cells are developed w/n testis)
5) With what other tissues must Gonocytes interact in order to function? Keep in mind not only proximal tissues, but also important distal interactions eg hormones produced by various glands. (example: Signalling – FGF-2/notch signalling in seminiferous epithelium; LH- aid for apoptosis; FSH- produce ABH by Sertoli cells.)
6) Outline aspects of cell-cell adhesion, proliferation, apoptosis and/or migration important for the development of this cell type (example: Sertoli cells, Leydig cells, Apoptosis- maintain ratio of Sertoli cells; SSC undergo proliferation.)
7) Provide some introductory information to your topic.( example: Cryptorchidism Definition, % affected, 1-2 lines relate to infertility and how male reproductive health needs attention.)
Topic – Cyptorchidism
This section should be around 3 pages long, and have a focus on the developmental biology of Gonocytes including images.
Few key points of how what should the discussion consist of: (can add in more).
• How Cyptorchidism relates to Gonocytes/primordial germ cells at least half a page
• Comparison b/w normal & UD testis; Mention in brief about Diseases (cancer), health conditions(diabetes) disorders (ADHD, ASD) can cause.
• No presence of BAXprotein, gonadotrophins (LH, TSH) – due to low sperm count not allow apoptosis/ self-renewal/high temperature in testis of Gonocytes.
• Importance of Mini-puberty
• Mention of surgery – orchiopexy in children to fix testicles
• Impact on infertility – comparing % Fertility rates between countries; Discuss the health conditions mentioned at above; how society ignores male reproduction.
Answer
Gonocytes
The report of Gonocytes assignment examines the several aspects of Gonocytes which are the precursors of spermatogonia. Before getting differentiated into spermatogonial stem cells, these can be found from week 7 of the embryonic development till the neonatal stage. Male germ cells serve as the mode of inheritance via transfer of epigenetic and genetic information between generations. Spermatogonia are central to fertility in males, which in turn depends on high population of stem cells. (1) Hence, it can be said that the quality and function and quality of a sperm cell depends on its origination, i.e. spermatogonial stem cell (SSC).
Germ cells are represented by Gonocytes during the migratory developmental stages which are short-term and successive. These stages happen from the time of inhibition of formation of gonads on the genital region by them till the time of their migration to the seminiferous cords’ basement membrane. The development of Gonocytes is divided into various phases: cell proliferation, differentiation, migration, and apoptosis. (2) Any abnormality during any of these stages may lead to some disease related to fertility.
Before settling into gonads, Primordial Germ Cells (PGC) undergoes proliferation several times in testes before being differentiated into gonocytes. The proliferation of gonocytes continues even after colonization, a burst of apoptosis happens in parallel to the proliferation. Cyclin B1 is a critical factor for the proliferation of gonocytes, which is required for a proper spermatogenesis. Various research show that a shortage of cyclin B1 during the postanatal stages leads to a decrease in the germ cells due to increased apoptosis and mitotic arrest. (3) Proliferation is inhibited in a majority of gonocytes when the spermatogonial stem cells after 17-days of conception and they resume mitosis after birth. During this phase as mentioned in the section of Gonocytes assignment, proliferative post-natal and fetal gonocytes die but no other gonocyte dies. Transforming Growing Factor ? (TGF?) signaling plays an important role in the spermatogenesis process as it is involved in the regulation of various processes linked to germ cells like apoptosis, differentiation and proliferation.
Interestingly, DNA-damage-inducible-45-alpha (Gadd45?) and growth arrest is expressed in germ cells in particular. Research on the case scenario of Gonocytes assignment has shown that along with somatic cytochrome c (Cycs), Gadd45? expression upregulates the differentiating gonocytes. Any deactivation in expression of these two genes may lead to uncontrolled proliferation, and proneness to cancer. These genes are also found to play an important role in the initiation of germ cell apoptosis and gonocytes differentiation.
Neonatal gonocytes proliferation is stimulated by the 17?-estradiol (E2) and paracrine fashion platelet derived growth factor (PDGF) which are synthesized and secreted by sertoli cells. PDGFR is involved in the proliferation and apoptosis modulation in neonatal gonocytes. (18) E2 acts as a pro-apoptic and an anti-mitotic agent for the neonatal murine gonocytes.
Spermatogonia from Gonocytes:
Gonocytes get relocated to the basement of testicular cords from the lumen after birth. Migration on one hand is required for proper differentiation to spermatogonia, while on the other hand it helps in establishing a strong interaction between pre-spermatogonia and Sertoli cells. After migration, apoptosis happens on the pre-spermatogonia which are left at the center of the cord.
A protein complex known as ADAM-integrin-tetraspanin complex is organized in a sophisticated complex of microdomain membrane known as the tetraspanin web. (17) The attachment of Sertoli cells and pre-spermatogonia is stabilized by binding of tetraspanin web by F-actin. This binding is such that upon detachment, it leads to fragmentation and structural changes of F-actin. Sertoli cells mentioned in this section of Gonocytes assignment clarifies that subsequently release Fas-ligand which is locally soluble and binds FasR in the pre-spermatogonium which is detached. (4)This in turn leads to apoptosis which is extrinsically induced. This apoptotic signaling is evaded by the gonocytes which have timely migrated to the basement membrane, mainly because of their new microenvironment. Hence, it can be established that there is an active communication between Setoli cells and germ cells. And the numbers of germ cells that are supported are guaranteed correct physiology through induced paracrine apoptosis.
Gonocytes are differentiated into type-A spermatogonia after birth. Interstingly in humans, the amount of germ celss are reduced to less than half. And by the end of 2 years, no gonocytes are left through the removal of non-differentiated gonocytes via apoptosis. In humans, by the age of 3-4, type-A spermatogonia get converted into Type-B spermatogonia, which mature into primary spermatocytes after migrating to center of testicular cord. (5)
The investigation carried on the Gonocytes assignment mentions that approximately 75 percent of the germ cells in a healthy male are redundant from spermatogonia to mature spermatozoa. In simpler terms, apoptosis bursts are primarily responsible for selection of such severity. From the above arguments, it can be deduced that the better the understanding of spermatogenesis and the development of the male germ cells, the higher is the potential for them to be used for infertile testis reconciliation. Apoptosis forms an essential part of the process of germ cells development, starting from early stages of embryo till the spermatogenesis completion. The fertility of the male and their delivery of perfect genetic message to the offspring is dependent on the apoptosis at right time and right place of gonads.
Results:
Effect of FGF on Testicular Sertoli and Leydig Cells
Throughout the testis, it is noted herein Gonocytes assignment that the FGFs gets localized to many cells, like Leydig, germ cells and Sertoli. DNA synthesis and cell multiplication, along with their phenotypic expression, have been seen to be stimulated due to the FGFs in cultured Sertoli cells of pig. Upon 3 to 6 days culturing of the cells in isolated 3-day-old, newborn or fetal rats, FGF-2 was found to increase the Sertoli cell numbers. FGF-2 displayed a survival factor for these cells in vitro as they did not lead to an increase in the Sertoli cells’ [3H] thymidine labeling index. (6)
The readings used in the context of Gonocytes assignment illustrates that FGF-2, as a mitogenic factor, may also be required during the immature testis development. Through various immune histochemical evidences, presence of FGF-2 in Leydig cells of fetus has been established. Purified cultures of porcine Leydig cells were taken from the animals, to form a basis of primary model in order to study the mechanism and effect of FGF-2 on steroidogenesis of testis. (16) FGF-2 was found to increase the accumulation of chorionic gonadotropin (hCG)-induced testosterone in human, in the intermediate to a long term treatment of the cultured Leydig cells. Its pleiotropic role is suggested strongly due to its ability to affect the maximal steroidogenic capacity of the Leydig cells.
It was also found in the studies utilized to prepare this Gonocytes assignment that the Leydig cell function’s regulation also involves factors that are locally produced. These factors also include the members of FGF family. Research on Leydig cells isolated from rats of various ages was done in order to understand the effects of FGF-1 and FGF-2. It was shown in the research that FGF-1and 2 stimulated the production of basal 17?-diol and 5?-androstane-3? through immature Leydig cells and production of Luteinizing Hormone-stimulated testosterone by fetal Leydig cells. (7) FGF-1 and FGF-2 had no impact on the production of testosterone by adult Leydig cells, but FGF-1 alone was found to inhibit the production of LH-stimulated testosterone by mature Leydig cells. The research on the case of Gonocytes assignment demonstrates that the effects of FGF-1 and FGF-2 are Leydig cell development and differentiation dependent.
Expressed widely in embryonic tissues, FGF-8 plays an important role in the morphogenesis of central nervous system, face and limbs, and elongation of body axis. But in an adult mouse, expression of FGF-8 m-RNA has been detected in the testis through Northern blotting. (15) Due to the decrease in the proliferation of murine Sertoli cells after birth and it ceasing while puberty, a very low level of FGF-8 expression can be expected in adult testis. The stage of expression of FGF-8 illustrated in this segment of Gonocytes assignment shows its specific function in the testis’ seminiferous epithelium’s maturation.
Figure: Immunofluorescent detection of expression of GFRA1 and FOXO1 protein treated without and with signal inhibitors. (6)
Effect of FGF on Testicular Germ Cells:
A wide range of cellular activities like cell differentiation and proliferation during embryonic development of various organs are regulated by FGFs. Fully functional sperm depends heavily on the testis’ environment and cannot develop on its own. Regulating the testicular cell growth is necessary for the spermatogenesis maintenance in the adult testis. The study examined in the Gonocytes assignment mentions that presence of specific growth factor is required for the high rate of germinal cell proliferation in the adult testis.
Paracrine regulation of the growth of testis germ cell also involves some members of FGF. FGF-2 supplement led to a significant increase in the gonocytes number, which were cultured for 6 days and separated from animals which were newly born or 3-day-old. (14) The germ cells’ numbers were found to be doubled as compared to the control cultures. Research have also shown that spermatogenic meiosis and mitotic proliferation of spermatogonia is stimulated by FGFs partially through FGFRs in order to enhance the activity of mitogen activated protein kinase (MAPK).
Figure: (A) GST characterization in the murine testes. GST is initiated in gonocytes. (B) GST regulation in the murine testes. FGF signaling is the basis of signaling mechanism. (4)
Discussion:
The phenomena of Cryptorchidism explored herein Gonocytes assignment is caused when an individual scrotum lacks of one or both the testes. This is one of the common defects in the genital tract of males from their birth. Around 1 to 4% newborn males suffer from undescended testis or Cryptorchidism which eventually leads to infertility and testicular cancer. According to the research, infertility in undescended testis (UDT) is due to the development of abnormal gonocytes that generally during minipuberty (in human 2-6 months and in mice 2-6 days) convert into spermatogonial stem cells (SSC) or undergoes programmed cell death. (8) Throughout minipuberty under the control of FSH ns indirect control of Androgen and LH the hypothalamic–pituitary axis stimulating gonocyte transform. An arrested gonocytes as well as developmental abnormalities in germ cells is the root cause for malignancy in later age of an UDT individual.
Development of the Germ cells:
Development of germ cells is an active process. After the birth (within 1st year) neonatal gonocytes starts changing into adult dark spermatogonis. These adult stem cells comprise a dark nucleus that differentiates them from the other cells. Adult dark cells does not take part directly in spermatogenesis instead make sure to supply a certain amount of stem cells required for spermatogenesis.Certainly, Adult dark spermatogonia replicate to produce light nuclei comprising adult pale (AP) spermatogonia.(13) By the mitosis process these cells are produce which further become primary spermatocytes by dividing and differentiating (found in 4 year boys).
According to several research data considered to develop this report on Gonocytes assignment, AD is formed in the age of 3 to 9 and this cycle requires an optimum temperature (330C) as well as normal amount of testicular hormones like androgens and gonadotropins. (9) All the neonatal gonocytes does not convert into AD spermatogonia instead undergo involution via apoptosis. Environmental and genetic conditions effect these processes.
Recently the development and modification of germ cell in cryptorchidism is been studied by several researchers. The process by which sperm cells are produced is known a spermatogenesis. During puberty it begins to form with the increase amount of testosterone and gonadotropins. This process involves various complex process in a sequential manner like first mitosis takes place and then followed by meiosis and differentiation. (12) In every steps autocrine, paracrine and endocrine factors participate. The inability to transform gonocytes into AD cells leads to infertility in males.
Figure: Presence of abnormal gonocytes in a boy of 6 months of age with cryptorchidism. The germ cells are indicated with mouse homolog of Drosophila Vasa and the Sertoli cells with MIS/AMH. (3)
As per the readings of the authors considered to develop the study within this Gonocytes assignment, the abnormal high temperature in the cryptorchid males is the main reason for the mal development of these germ cells.
Significant studies observed in various animal models suggest that due to heat stress both direct and indirect effects is applied on the germ cells, initiating impaired transformation, maturation of the cells and inhibiting apoptosis. (2) This injury is caused by heat shock protein and certain reactive oxygen species that damage the Sertoli cells and the germ cells.
Disease associated with cryptorchidism:
Researchers have observed that the precursor cells of testicular cancer are alike to fetal gonocytes. According to a recent study used to develop this Gonocytes assignment, the abnormal high temperature of UD leads to an irregular apoptosis that let some gonocytes to remain arrested and transform in cancerous cells that leads to malignancy and infertility in adulthood via several mutations and cellular unbalance.
Figure: Spermatogenesis and development of cancer: Malignant and normal testicular germ cell development.
The established etiology for germ cell carcinoma is still unknown though instabilities in the microenvironment delivered by the Leydig and Sertoli cells plays an important role. In fact this process of spermatogenesis is influenced and controlled by various signaling pathways those are delivered from the Leydig cells and the local environment. Throughout the development process expression of the insulin-like-3 gene (INSL3)is visible that is responsible for testicular descent and gubernaculum maturation.(11) It has been observed that there is a reasonable relationship in between INSL3 and cryptorchidism males.
What is the role of treatment performed in Cryptorchidism in the context of Gonocytes assignment?
Orchidopexy is one of the most common practice in cryptorchidism treatment. Other two approaches are inguinal operation and laproscopy. (10) Initial surgical treatment may avoid infertility. Orchidopexy surgery is mostly perform before the age of 2 and several recent studies suggest that if this surgery is done before the age of 1, normal spermatogenesis may achieve by inhibiting the progressive changes of the testes and germ cell loss. Though this surgery does not assure normal fertility in later life. According to Hadziselimovic, that despite performing orchidopexy before the age of 6, nearly 35% of boys were found to be infertile irrespective of the total normal germ cell count.
Mini Puberty:
A period in infancy when male fertility recognized (30-90 days after birth) is known as mini puberty. (10) During this puberty period there is a temporary increase in certain hormons like testosterone and gonadotropins that helps the gonocytes to diferenciate into AD germ cells.This AD spermatogonia establish germ cell memory and pathways of DNA methylation specific to the males. According to several studies it has been found that there is a mild primarily disfunction in the boys having cryptorchidism. It is observed herein Gonocytes assignment that early after birth there is a deficiency of androgen and testosterone indicating a disturbing function in the testicles. This hormonal deficiency in 3-month child shows lower amount of production in inhibin B.
Nonetheless, boys having cryptorchidism as found to have low levels of testosterone, luteinizing hormone (LH), abrogated variation of Ad spermatogonia from gonocytes and atrophic Leydig cells. The major difference between the UDT and normal children is the reduce amount of response to human chorionic gonadotropin (hCG) by the leydig cells. Early treatment with hCG in UDT boys can strike out the difference in terms of stimulation. (5) Therefore, the reason behind lower response of testosterone obtained in this aspect of Gonocytes assignment appears to be at the hypothalamic level and perhaps due to insufficient stimulation by the Leydig cells. Several LH-RH tests have confirmed a lower Luteinizing Hormone response by gonadotropin-releasing hormone.
A Normal separated germ cells in newborns with mini-puberty. One Adult dark and two Adult pale germ cells are present. The gonocyte cells is missing. B Compromised mini-puberty leads to a defective conversion of gonocytes into Adult dark spermatogonia. Atrophic Leydig cells are observed between the tubles which proves the presence of impaired gonadotropin stimulation.
Ignorance of society about Male Reproduction:
According to the human fertilization and Embryology Authority report (2014-16) observed in the context of Gonocytes assignment, male infertility (around 37%) is a common problem in recent times. Meanwhile this area of research does not get more priority. This is because of the bizarre and deep rooted perceptions of the society about importance of femaleness in infertility. The way that these problems, pain, ineffective treatment, infertility and ultimate responsibility or blame are often being associated with females is not only a female issue but also a failure of female. On the other hand, with males this issue is a shooting blank. This complex biological and cultural perception of the society towards the females leads to violence and even criminal incidences. According to the research performed by Harvard Medical School in Boston, it is noted herein Gonocytes assignment that the sperm quality of the males reduces with age and create troublesome in fertilization and also potentially affect the infants’ health. Despite the fact even today men can fail in innumerable ways as per the society but a woman is considered as messing up everything and the total blame is put on her of not being able to bear a child.
References
Research
Artificial Nanoparticle Blood Instant Oxygen Boost Report Assignment Sample
Question:
Prepare a basic business model for Artificial Nanoparticle Blood – Instant Oxygen Boost
Answer
Introduction
The research discusses the Artificial Nanoparticle Blood-Instant Oxygen Boost, which uses nanoparticles to control the hemoglobin’s oxygenation while allowing for the logical design of oxygen carriers based on hemoglobin. The oxygenation properties of adult human hemoglobin and sickle cell hemoglobin, which are linked to an increased oxygen affinity, are retained during the adoption process. The hemoglobin tetramers are adsorbed on the surface due to the hemoglobin's strong affinity for nanoparticles (Andocs et al., 2015). As a result of the interaction between nanoparticles and hemoglobin at the molecular level and its effect on the qualities defined for the oxygenation process that are precisely regulated, the development of bio-nanotechnology opens the door for the construction of blog replacements.
Value Proposition and Customer Segment
The customer segment is based on comprehending the needs of patients with problems, which can be separated into infectious and non-infectious concerns. This study shows that different users are used in different ways by all users, including interested parties, investors, consumers, analysts, reviewers, and suppliers. Stakeholders are connected to a number of suggestions and proposals for enhancing the auditors' report. They recognize concepts and strategies that could be vastly dissimilar from those of auditors and strengthen control over financial statements (Gy et al., 2015). It is common for all users to gain from an analyst's effort to forecast future sales, which usually indicates that other users, particularly those taking part in the evaluation, are also included. Accounting analysis is a typical account since it appears on the balance sheet; it is a real account with a recurring revenue stream. Controlling the exceptional preservation of the qualities of human adult and sickle cell hemoglobin depends on understanding the molecular interactions between nanoparticles and hemoglobin as well as their impact on the oxygenation properties. These real accounts are therefore likely to be balances that should have been erased over time without proper accounting investigation. It is possible to create an accounting examination of nominal accounts that includes a profit and loss account or with the help of biology dissertation help online. Accounting analysis is connected to financial statements that assess an organization's capacity to fulfil its obligations to recipients and other third parties as well as its capacity to boost revenue (Jamshidi t al, 2015). Understanding configuration patterns with financial data that have been established to work with various data partition formats is crucial for performing proper data analysis. In this situation, the corporation should concentrate on securing the benefits of creditors or investors in order to acquire various vital assets.
Key Partners / Stakeholders
As a result, the financial condition of the Organization in relation to its competitors is taken into account in the study of the business plan.
Since financial statements satisfy all customer needs, the company has several users of them (Zeng et al., 2016). With the rise of HIV, which is mostly caused by the issue of blood transfusions that tend to impose greater expenses at the time of detection tests, fake blood is brought to light. The less plentiful blood is primarily used in poorer nations. The study is about the increase in the product's half-life due to the reduction of phagocytosis, and the enzymes are contained in Nano capsules with a membrane constructed of ultrathin polyethylene glycol acid. In order to generate nanoparticles and cell-like things that can usefully be able to improve the primary functions of red blood cells, various macroscopic selection that is decided by the endeavor to create good open access reviews must be managed. The difficulties are caused by changes in the blood's and the oxygen transport system's capacity (Hafsi et al., 2017).
The widespread synthesis of cells or entities that resemble cells is included in this conduct of oxygen-bearing nanoparticles. Users of the company's financial reports include:
• Investors: As they are owners of the company and are required to understand how financial resources are used, investors must be aware of the financial facts precisely (Kiransan et al., 2015).
• Management: In order to determine the company's functional and financial results, management must calculate the company's profit and cash flows on a monthly basis.
• Creditors: Lenders are interested in the cash flow and the minimal institution. They could receive loans from the business. Companies that are unable to repay loans put creditors at risk.
• Owners: Owners are typically the most significant customers and are interested in financial reporting. However, owners are also interested in profit and a fund where they must get a certain income and can participate. in the income statement's definition. Owners desire to know how much capital the business has used up so they can profit from sales (Ghaedi et al., 2015).
Key Activities and Resources
The financial situation of the company serves as the primary informational source in financial analysis. This information is required for the financial researcher's purposes. It is related to financial reporting since it organizes the assessment of the company's competitive work and determines the planning and operational effectiveness of the business (Dzamukova et al., 2015). The performance of the company can be determined and standardized with the aid of financial analysis. We will also take into account how this may affect the analytical techniques used to analyses financial data. A balance sheet is used in financial analysis, which mainly serves to record changes and support the study of various obligations and capital. Double accounting and the application of generally recognized accounting rules define financial characteristics. Deliveries in this situation are dependent on liabilities with financial responsibilities, and they subsequently transfer other assets to offer effective services. Commitments rely on a crucial necessity to match the company's diverse assets. The share capital and net assets, which are calculated for management and participation in the share capital, are merged for long-term employers. Work to keep the profits that the company is currently making by reinvesting them back into the company. These templates are based on payments made to the business's owners, in which the parties concerned and profits also govern the amount of retained earnings. The application is based on the shareholders' dividend payments, which may coincide with an offer of a certain amount of money.
Customer Relationships and Channels
The fundamental issue is that CRM places a lot of emphasis on knowledge and various geographic boundaries. The situation is regarded as vital and as being in conflict with the circumstance. Social philosophers hold the views that are typically sought after by those and others who view themselves as distinct from the natural sciences. Real-world lifestyles are based on observations made by people with an interest in them. Here, divers prepare for various scenarios, and special factors serve to ascertain the public's overall understanding by a particular study. His study of corporate governance takes a pro-active stance in the pursuit of cause-and-effect relationships that establish system norms, objectives, and hypothesis tests. In order for the nanoparticles to carry more oxygen and eventually reach the body's tissues, it is critical to assess their engineering. Here, the treatment is determined by the injuries and whether the best oxygen-carrying nanoparticles are deemed secure over the long term.
Cost Structure and Revenue Stream
One of the financial statements' drawbacks is its reliance on historical costs when analyzing the balance sheet's various activities and asset and liability values. They frequently vary along with marketable securities, which are primarily modified to reflect shifts in marketing values. Concerns exist over the division's handling of unrecorded intangible assets in relation to the division's products, services, clients, and customers. The rule is to manage the commercial value and focus on a certain time period strategy.
In cases where the cash flow of the company is best understood by carefully examining one of the reporting periods, the use of financial statements can result in an inaccurate interpretation of the financial results. The management team's exposure to fraud activities while working with skew outcomes could result in a situation where other good results are exposed to undue pressure when it comes to bonus plan payouts. The management of business operations and the development of system standards with non-verification methods that are connected to measuring the issuer's policies, practices, and controls have not been discussed. The data must be set with the financial statement that offers the forms for system processing and the various variable costs that must be efficiently managed.
Revenue Streams
These are the sources of income, or revenue streams. Here, you should emphasize any potential sources of funding for the project:
• Insufficient Information: The financial statements are primarily used to determine the reporting period. As a result, there is a great chance of spotting inaccurate information. As a result, when the firm has ended, it is possible to determine the precise financial situation and the precise capacity of the organization's funds.
• Unlimited Information: To prepare financial accounts, accounting principles, practises, and conferences are used. Recruitment participation is also put to use for training. These outcomes are closely related to the financial accounts' correctness.
• The quality of information is neglected: The term "financial conditions" only refers to quantitative data, which is only included in financial reports. Quality facts, such as manager effectiveness, company generosity, employee-owner relationships, employee ability, and employee happiness, are completely ignored and are not referred to as financial terms. Therefore, in order to take a real financial condition and produce commercial results, these abilities are required.
• Incorrect disclosure of financial position: The company's financial status will be impacted by a number of factors, including commercial, state, and public finance, but only the financial part is taken into account in financial reporting. The financial statements do not include any commercial or general components. The company's financial status is disclosed as a result of this kind of routine practice.
• Financial statements are not specific: Financial reporting is not identified because numerous accounting methods are accessible to numerous organizations, accounting rules and regulations change, and the business capabilities of the companies vary from one another. Financial firms are unable to distinguish themselves as a result.
• The actual profit is not disclosed in the income statement: Operating and non-operating expenses are recorded in the income statement; the actual profit is not reported there. Only the assessment is presented; actual costs are not. The likelihood of withholding the true profit at the expense of profit and loss is then very high.
Conclusion
The company's interest is in better financial information management, particularly to draw in people and innovators who have debt with an interest rate. The interaction between Nano particles and haemoglobin at a specific level has been one of the other draws for the haemoglobin-based oxygen carriers. The primary purpose of using nanoparticles is to modify structural particles while releasing free haemoglobin into the bloodstream.
References
The following sources are cited: Andocs, G., Rehman, M.U., Zhao, Q.L., Papp, E., Kondo, T., and Szasz, 2015. Part II of nanoheating without synthetic nanoparticles. Utilizing an experiment with the U937 cell suspension model, the modulated electro-hyperthermia method provides support for the nanoheating notion. 7(4), page 1 of Biology and Medicine.
2015 study by M.R. Dzamukova, E.A. Naumenko, E.V. Rozhina, A.A. Trifonov, and R.F. Magnetic nanoparticles supported by polyelectrolytes are a simple method for creating artificial multicellular tissue-like clusters for cell surface engineering. Pages. 2515-2532 in Nano Research, 8(8).
2015. Artificial neural network-based genetic algorithm based optimization for the adsorption of phenol red (PR) onto gold and titanium dioxide nanoparticles loaded on activated carbon. Ghaedi, M., Daneshfar, A., Ahmadi, A., and Momeni, M.S. 21, pp. 587–598 in Journal of Industrial and Engineering Chemistry.
(2015). Gy, V., Szigeti, G.P., Andocs, and Szasz without the use of synthetic nanoparticles, nanoheating The following authors published a paper in 2017: Hafsi, B., Boubaker, A., Guerin, S. Lenfant, S. Desbief, F. Alibart, A. Kalboussi, D. Vuillaume, and K. Lmimouni. Artificial synapse using electron-transport polymeric gold nanoparticles for neuromorphic applications. 499–506 in Organic Electronics, 50.
The following authors published a paper in 2017: Hafsi, B., Boubaker, A., Guerin, S. Lenfant, S. Desbief, F. Alibart, A. Kalboussi, D. Vuillaume, and K. Lmimouni. Artificial synapse using electron-transport polymeric gold nanoparticles for neuromorphic applications. 499–506 in Organic Electronics, 50.
Karaca, S., Sheydaei, M., Khataee, A., and Kranan, 2015. ZnO nanoparticles produced on montmorillonite are used in the photocatalytic removal of a dispersion dye in artificial neural network modelling. Molecular and Biomolecular Spectroscopy, 140, pp. 465–473, Spectrochimica Acta Part A.
A 2016 study by Zeng, H.H., Qiu, W.B., Zhang, L., Liang, R.P., and Qiu, J.D. Lanthanide coordination polymer nanoparticles serve as a superior synthetic peroxidase for the detection of hydrogen peroxide. Pages 6342–6348 of Analytical Chemistry, 88(12).
Case Study
Old Man with Pneumonia Case Study Assignment Sample
Question
Task: This assessment task gives you the chance to read extensively about the health status of a critically ill patient. You must choose a case for analysis that either had shock, acute respiratory failure, or heart failure. We are looking for evidence of at least one management strategy that has been presented, as well as a demonstration of a thorough comprehension of the applied pathophysiology that supports the patient's primary condition.
Please be aware that we are not searching for extensive information about the patient. The message can be communicated in one or two sentences (see the example). Since no patient information would be presented in the manuscript, institutional approval is not necessary. It is not sufficient to just list test results if you desire to incorporate them; a list does not express your opinion and does not thus contribute to the grading. Instead, you must offer the test findings in the context of your analysis. You could say, for instance:
The patient's symptoms included hypotension (mean arterial pressure of 48), hypoxia (low oxygen saturations), acidosis (pH of 7.16), and hypocapnia (55 mmol/L of carbon dioxide).
In terms of case selection, if you feel as though you are at a loss for what to choose for review, please speak with your instructor to go over your possibilities. Heart failure is not an acceptable topic choice for cardiovascular students. Positive pressure ventilation cannot be discussed by intensive care students.
Examples of instances and subjects for review
It is not necessary for the subject or case to be complex. Simple examples include
• lowering of blood pressure
• decreased oxygen saturations
• the vasovagal episode
• gastric contents being aspirated
• pneumonia
• hypertension
and so forth
No of the subject, it is your responsibility to describe the compensating processes that are engaged in reaction to the issue. Then you make at least one management strategy a link to the associated physiology.
Answer
1. Introduction:
The pathophysiological issues of James, a gentleman of 57 years old, will be critically discussed in the pneumonia case study. Due to abrupt hypoxemic respiratory failure brought on by severe pneumonia, he was sent to the intensive care unit (ICU) (Mansour et al., 2015). After seeing Japan and New Zealand, James had just returned from a two-week trip abroad. James had been healthy for the 21 days before to admission and had just recently started experiencing modest symptoms. However, two days ago, his condition quickly deteriorated, leaving him with a 39.2-degree fever, shivering, hypoxia, arthralgia, myalgia, and persistent shortness of breath. Since the patient needs some supportive care and has been diagnosed with influenza. Additionally, the acute clinical management strategy for the pneumonia case study will be critically analysed. James will receive additional tests and chest X-rays as his condition worsens in the intensive care unit, and it will be determined whether the clinical management plan is supported by the most recent evidence-based literature.
2. Ethical Use of Patient Healthcare Data:
In terms of ethics, the patient is responsible for allowing the use of their data in research studies. When any type of research is conducted using patient data, ethical problems typically surface. To ensure that patient data is evaluated ethically and that outside assistance is only sought when necessary, it is the patients' and the healthcare department's obligation.
3. Pathophysiology Problem and Treatment Analysis
The human respiratory system typically involves the passage of air through the nose, nasal cavity, pharynx, larynx, trachea, and bronchi after passing through the nose and nasal cavity (Luo, 2016). The Nares is the name of the aperture to the nose. The Nasal cavity has a mucous membrane lining it, along with cilia and blood vessels. The proposed Biology case study shows that there is a cilia where some air filtration is done, warmed by the blood, and moistened by the mucous membranes. A portion of the throat, sometimes known as the pharynx, is where air passes. Williams and Tordoff (2018). The food and air typically go along this route. The air then keeps moving through the larynx. The epiglottis is a flat piece of tissue that is visible on the other side of the larynx and is closed when swallowing anything to keep it from getting into the alveoli. A vocal fold is located in the larynx, or voice box. The bronchial tree and larynx are joined by the windpipe, often known as the trachea. To keep the trachea from collapsing, it features a cartilage ring. The pneumonia case study investigates the fact that the lungs' spongy tissues are also connected to blood capillaries and alveoli in the respiratory system. Human bodies begin to breathe when their lungs dilate and then expand. The smaller bronchi, known as bronchioles, are carried by the air through larger bronchi. At the ends of each bronchiole, there are alveolar sacs. The Alveolar Sacs have blood capillaries that surround them, contain a single layer of millions of alveoli cells, and carry out gas exchange functions. When air is inhaled through the nose, oxygenated air first travels through the pharynx before moving through the larynx, trachea, bronchi, and finally the alveoli. When capillaries process the fusion, oxygen from the air is transferred to the alveoli. In order to exhale carbon dioxide, the capillaries push the gas to the alveoli. Respiration is the name given to such a procedure.
The exchange of gases between the bodily cell and the atmosphere occurs during respiration. In it, numerous things happen, one of which is breathing. The Alveolar Sacs transport air to the Alveoli, where gas exchange is processed and easily absorbed (De Giacomi et al., 2018). Alveoli perform the gas exchange between epithelia capillaries and alveoli when they are filled with air during an inspiration. The blue gas over here is the carbon dioxide that has been exhaled, and the white gas over here is inspired oxygen. Red blood cells ultimately change colour after a gas exchange is completed. The red blood cells are altered in the capillaries such that the alveoli release carbon dioxide before binding oxygen.
An inflammation in the lower respiratory tract and a deadly infection were discovered in a case study of a patient with pneumonia. This infection is brought on by pneumococcus streptococcus pneumonia, which is a combination of a virus, bacteria, mycoplasma, and pathogens that can be breathed. The infection increases the amount of fluid that is secreted, and this extra fluid pools and gathers in the lungs' air sacs (Dumas et al., 2019). Pneumonia is associated with the air space in the lungs when excaudate is present, which produces coughing, acute chest discomfort, shortness of breath, and a high temperature. When the patient was first admitted to the hospital, the same situation was seen in him as well (Dickson et al., 2017).
The next day, an x-ray of the chest revealed pneumonia in the left upper lobe. Due to his acute hypoxemic respiratory failure, the patient was prescribed an albuterol inhaler and azithromycin. He also received an intravenous stat dose of ceftriaxone and azithromycin before being discharged and allowed to return home (Ramos-Rossy et al., 2018). After two days, the patient began to have chronic symptoms, for which cough syrup and prednisone were recommended. The patient from the pneumonia case study reported exhaustion. His temperature had risen to 38.8°C. Since he took acetaminophen, he was unable to fall asleep. He also had a persistent cough that occasionally produced blood-tinged sputum, as well as pleuritic chest pain. He had dyspnea when I was assessing his health (Kazzaz et al., 2017). As a nurse, I evaluated the patient's vital signs and discovered that their blood pressure was 148/72 mm Hg, their breathing rate was about 26 breaths per minute, their pulse rate was 88 beats per minute, and their oxygen saturation level was 94%. Some crackles had been observed in the left axillary region and left lung base (Serota et al., 2018). A significant infiltration is visible on the left side of the chest radiograph.
The left upper lobe of the chest was observed to have a severe consolidation on the lateral and posteroanterior chest radiographs, whereas the right upper lobe had a mild consolidation and a faint left pleural effusion. Lymphadenopathy was absent, nevertheless.
Prior to admission, a portable anteroposterior via the chest radiograph was taken to check for more lung opacification. The left upper lobe showed the most involvement, and the right upper lobe of the right lung showed multifocal consolidation (Sanz-Herrero et al., 2016).
The doctor started a Vancomycin therapy on the third day, and on the fifth day, he started an imipenem therapy. However, the patient developed hypoxemia on the sixth day, for which he required four litters of oxygen therapy delivered through nasal prongs. Around 270 mg per deciliter of?1-antitrypsin was present (Kawakami et al., 2019). IgM, IgG, and total bilirubin levels in the blood as well as their anion gaps were all within normal ranges.
According to the readings generated in this case study on pneumonia, the patient was transferred to the intensive care unit (ICU) for additional assessment and maintained there for two days. A bronchoscopy was performed, and the results showed a purulent discharge without any mucous plugs. The left ventricular's ejection fraction ranged from 60 to 65%. The cultures of the sputum samples were unfavourable (Kushwah et al., 2018). It was discovered that the echocardiography had biventricular function and wall motion.
By the ninth day, the airway pressure that was prescribed to have positive bi-level had gradually improved. Methylprednisolone was given at the precise moment the trachea was intubated voluntarily on the same day. The sputum cultures had produced species of candida while the blood cultures came back negative (Park et al., 2016).
The patient needs to be admitted to the ICU once more on day ten. Norepinephrine, fentanyl, and midazolam intravenous infusions, as well as moxifloxacin, vecuronium, vancomycin, imipenem, ondansetron, sodium succinate, acetaminophen, pantoprazole, enoxaparin, ondansetron, albuletrol, nystatin suspension, and fluticasone nasal spray, were among the medications the doctor The patient also had indomethacin for his gout and received an influenza vaccination. The administration of drugs revealed no allergy symptoms in his body, nonetheless. He informed me about himself and the places he had lived and worked as I was watching him. He also revealed that he used to smoke 40 packs of cigarettes every two weeks, but for the past six months, he had quit before falling ill and being brought to the hospital (Ivanick et al., 2019). He doesn't use any illegal substances, and he used to drink alcohol very infrequently or on special occasions.
His mother and sister both had a?1 - antitrypsin deficiency, and his mother also had chronic obstructive lung disease, it was discovered when he spoke about his family history (Hirai et al., 2017). At the age of 63, his father passed away from a myocardial infarction. He was afebrile, sedated, and intubated as the patient studied for the case study on pneumonia was being examined. His heart rate was 50 beats per minute, his respiratory rate was 38 breaths per minute, and his blood pressure was measured at 89/22 mm Hg. He received ventilator assistance during this stage (Orsini et al., 2020). It had normal heart and bowel sounds, but there was considerable swelling in the limbs and legs indicative with 2+ edoema, as well as bilateral breathing noises. Plasma anion gap and serum protein electrophoresis were both found to be within normal ranges. hazy looking urine After urine examination, it revealed that there was 1+ albumin and 2+ urobilinogen (Yoshimi, Satou and Mori, 2018). Additionally, according to his report, he has 3 to 5 white cells, 5 to 15 red blood cells, very few renal tubular cells per high power field, few squamous cells, few reparative cells, 30 to 90 granular casts per low-power field, 20 to 100 hyaline cast, and mucus (Mangioni et al., 2019).
It is necessary to insert the esophagogastric and endotracheal tubes in the chest radiography that was shown in the pneumonia case study scenario. Additionally, there was a significant rise in bilateral multifocal consolidation without pleural effusion and pneumothorax. The oxygen inspired has a proportion of oxygen growing toward 1, the arterial partial pressure of oxygen (PaO2) rises to 93 mm Hg, and the oxygen saturation is 95%. (Wang et al., 2018). He received infusions of fentanyl, propofol, vasopressin, meropenem, and methylprednisolone. Later, I discontinue delivering imipenem. When the sputum sample was stained with gram's solution, it became apparent that the polymorphonuclear leukocyte count was moderate. The test came back negative for adenovirus, respiratory syncytial virus, and parainfluenza antigens (Furumido et al., 2019). Nucleic acid testing was done for the influenza A and B viruses.
A two-drug antifungal chemotherapy regimen is necessary for the patient's treatment. As part of the treatment, corticosteroids will be given. Exercise-induced hypoxemia and restrictive ventilator dysfunction continue a year after therapy is finished.
4. What conclusions were drawn from the investigation on the pneumonia case study?
Pneumonia is quite common in people between the ages of 66 and 88, and it's more likely to be acquired in the community, according to one of the research taken into account when creating this case study on pneumonia (Sonaglioni et al., 2019). Another study indicated that approximately 14,069 Medicare recipients older than 65 who had a severe case of community-acquired pneumonia were admitted to the hospital. The geriatric age group difference was between 78 and 8 years, according to a different study used to construct this case study on pneumonia (Kwon et al., 2018). This could be as a result of the varying older population's hospitalisation rate and access to healthcare. Most cases of pneumonia in elderly patients are brought on by the lungs' loss of elastic recoil, the mechanical clearing of the airways, the weakening of the respiratory muscles that cause coughing, the aging-related decline in mucociliary clearance, the cumulative effects of coexisting chronic diseases, defects in cell-mediated immunity, and humoral immunity (Prendki et al., 2018).
When analysing the cases with sex distribution to support the pneumonia case study scenario, it is found that more males than females are affected, with the percentage of females being around 30% (lès-Nancy and Vandoeuvre-lès-Nancy, 2019). The fact that smoking and drunkenness are more prevalent among men may be to blame for this. Additionally, it could be a result of worsening COPD or heart failure.
5. Conclusion
According to the critical examination of the case study on pneumonia, the condition is a major issue for older individuals and is frequently encountered in clinical settings. The most typical signs of pneumonia in older patients include respiratory symptoms, gastrointestinal symptoms, tachypnea, tachycardia, and crepitation. When collecting sputum from an old patient, it is frequently difficult to pinpoint the etiological agents. The process of isolating the responsible microbes might occasionally be complex. Empirical therapy may therefore be required in this situation. The results of the pneumonia case study show that environmental factors, such as climate, play a major role in raising the risk of pneumonia, which affects the respiratory system and causes inflammation and infection of the alveolar spaces in the lungs.
References
1. De Giacomi, F., Ryu, J.H., Yi, E.S., and Vassallo, 2018. eosinophilic pneumonia acute. the diagnosis, treatment, and causes. Pneumonia case study American journal of respiratory and critical care medicine, 197(6), 728–736.
2. Immunocompromised Pneumonia, 2017 by R.P. Dickson. The Critical Care Evidence-Based (pp. 215-220). The Springer, Cham.
3. Pène, F., Kontar, L., Bruneel, F., Klouche, and Barbier, 2019. Dumas, G., Demoule, A., Mokart, D., Lemiale, V., Nseir, S., Argaud, L., and F. Acute hypoxemic respiratory failure in critically ill immunocompromised patients: centre effect in intubation risk. 23(1), page 306 of Critical Care.
4. It was published in 2019 by Furumido, M., Takamura, K., Nakakubo, S., Kikuchi, H., Yamamoto, M., and Kikuchi, K. Pseudomonas aeruginosa Was Found to Be the Cause of a Case of Fulminant Type Community-Acquired Pneumonia at Autopsy, Archives of Clinical and Medical Case Reports, 3, pp. 451-456.
5. Yamagishi, Y., Mikamo, H., Haranaga, S., Kinjo, T., Hashioka, H., Kato, H., Sakanashi, D., and Fujita, 2017. Daptomycin-induced eosinophilic pneumonia: six instances from two institutions and a review of the literature. 23(4), 245-249, Journal of Infection and Chemotherapy.
6. 2019 Ivanick, N.M., Moh, M., Seeley, E.J., and Benn Endobronchial masses on both sides and severe hypoxemic respiratory failure. Journal of Bronchology & Interventional Pulmonology, 26(4), pp. e65–e67, case study on pneumonia.
7. It was published in 2019 by Kawakami, H., Miyabayashi, T., Tsubata, C., Ota, K., Ishida, T., and Kobayashi, O. A case series on the spontaneous remission of organising pneumonia brought on by thoracic radiation therapy. Case report on pneumonia in respiratory medicine, volume 26, pages 180–184.
8. N.M. Kazzaz, A.M. Wilson, R. Kado, G.D. Barnes, and J.S. Knight, 2017. A 37-year-old man with primary antiphospholipid syndrome who was experiencing breathing problems and developing toe ischemia presented. The arthritis journal, 69(8), 1253.
9. 2018; Kushwah, M.S., Verma, Y.S., and Gaur, A. Clinical indicators of hypoxemia in children with pneumonia recognised as such by the WHO. Int J of Contemp Pediatr, 5(4), pp. 1178–82.
10. 2018: Distinguishing Respiratory Features of Category A/B Potential Bioterrorism Agents from Community-Acquired Pneumonia. Kwon, E.H., Reisler, R.B., Cardile, A.P., Cieslak, T.J., D'Onofrio, M.J., Hewlett, A.L., Martins, K.A., Ritchie, C. and Kortepeter, M 16(4), 224–238. Health security
11. F. lès-Nancy and F. Vanduvre-lès-Nancy, 2019. Varnish Particle-Induced Acute Eosinophilic Pneumonia: A Diagnostic Challenge. Journal of Investigative Allergy and Clinical Immunology, 29(1), pp. 46–83.
12. In children with severe pneumonia and respiratory failure after NCPAP therapy, Luo, Y. 2016. Assessment of blood gas analysis results and degree of infection. 22(18), pp. 52–55 in Journal of Hainan Medical University.
13. Muscatello, A., Gori, A., Abbruzzese, C., Mangioni, D., and Bandera, A. 2019. asthma case study Intravenous immunoglobulins that are specific to the varicella zoster virus are used as an adjunctive therapy for severe varicella pneumonia. Infectious Diseases International Journal, 85, pp. 70–73.
14. 2015 study by Mansour, M.K., Ackman, J.B., Branda, J.A., and Kradin, R.L. Case 32-2015: A 57-year-old man who had severe pneumonia and hypoxemic respiratory failure. asthma case study 373(16), pages 1554–1564 of the New England Journal of Medicine.
15. J. Orsini, H. Gawlak, V. Sabayev, K. Shah, L. Washburn, K. McCarthy, A. Courey, E. Mouyeos, and S. Pangallo, 2020. Phlegmonocystis jiroveci Pneumonia-Associated Acute Respiratory Distress Syndrome in a Patient with Non-Human Immunodeficiency Virus Infection Complicated by Pneumomediastinum and Pneumopericardium 12(3), pages 209–213, Journal of Clinical Medicine Research.
16. 2016 publication by Park, D.W., Lim, D.H., Kim, B., Yhi, J.Y., Moon, J.Y., Kim, S.H., Kim, T.H., Shon, J.W., Yoon, H.J., and Shin, D.H. A case report and literature analysis on extracorporeal membrane oxygenation for acute respiratory distress syndrome after HAART Initiation in an HIV-infected patient being treated for severe Pneumocystis jirovecii pneumonia. ?????????, 31(2). (2).
17. As of 2018, Prendki, V., Scheffler, M., Huttner, B., Garin, N., Herrmann, F., Janssens, J.P., Marti, C., Carballo, S., Roux, X., Serratrice, C., and Serratrice, J. An interventional, prospective cohort study examined the use of low-dose computed tomography to diagnose pneumonia in older adults. 51(5), p. 1702375, European Respiratory Journal.
18. A study by J. Ramos-Rossy, J. Flores, Y. Otero-Domnguez, J. Torres-Palacios, and W. Rodrguez-Cintrón was published in 2018. failure of the respiratory system due to hypoxia brought on by zika virus infection. Journal of Puerto Rico Health Sciences, 37, pp. 99–101.
19. Gimeno-Cardona, C., Tormo-Palop, N., Fernandez-Fabrellas, E., Briones, M.L., Cervera-Juan,., and Blanquer-Olivas, J., 2016. asthma case study The potential benefit of the 13-valent pneumococcal conjugate vaccine in avoiding respiratory issues in pneumococcal community pneumonia. 34(15), 1847–1852, Vaccine.
20. 2018 March, Serota, D.P., Sexton, M.E., Kraft, C.S., and Palacio Acinetobacter baumannii-caused severe community-acquired pneumonia in North America: case report and literature review. Open forum infectious diseases case study of pneumonia (Vol. 5, No. 3, p. ofy044). Oxford University Press (US).
21. Anzà, C., Sonaglioni, A., Lombardo, M., Rigamonti, E., Vincenti, A., Nicolosi, G.L., Trevisan, R., Zompatori, M., Cassandro, R., Harari, S., 2019. A patient with hypertrophic cardiomyopathy and bilateral pneumonia presents with an unusual presentation of acute pulmonary hypertension. 20(12), pages 853–856, Journal of Cardiovascular Medicine.
22. A. Tordoff and L.A. Williams, 2018. Adults with community-acquired pneumonia: The diagnostic validity of physical examination procedures and the academic dissemination of these procedures.
23. 2018; Wang, Y.; Zhao, S.; Du, G.; Ma, S.; Lin, Q.; Lin, J.; Zheng, K.; Zhang, G.; Matucci-Cerinic, M. A case report and literature analysis describe acute fibrinous and organising pneumonia as the initial manifestation of primary Sjögren's syndrome. 37(7), 2001–2005, Clinical Rheumatology
24. 2018; Yoshimi, M., Satou, Y., and Mori, M. sputum cytodiagnosis revealed a case of herpes simplex virus pneumonia. Case report on a case of pneumonia, Clinical Case Reports, 6(1), p.
Essay
MIC11108 Drug Delivery System Assignment Sample
Extended essay guidance
What is the extended essay?
The extended essay provides an opportunity to conduct independent research or investigation on a given topic. The topic for this year’s assessment is:
A major challenge for the use of therapeutic agents is their efficient delivery to cells and tissues in the human body. Discuss currently available delivery systems and comment on the advantages and disadvantages of each approach”.
How long should my essay be and what font should I use?
Your essay should be a maximum of 2500 words (+/- 10%) excluding figure legends and bibliography. Essays should be typed using Ariel font, size 11, single spaced.
How should I structure my extended essay?
1. Introduction
Provide a general introduction to the topic. You might want to include:
i) What characteristics are desirable in a drug delivery system?
ii) What are the major challenges for drug delivery systems?
iii) Why is there a need for new effective drug delivery systems?
2. Main body
Provides a detailed description of selected drug delivery systems. Drug delivery systems are to be selected and researched independently by each student. I recommend that you describe 3 different drug delivery systems in your essay, however, if you want to focus on one or two delivery systems and carry out a more in-depth investigation, that is also acceptable. You might want to include:
i) An overview of the technologies i.e. how does the drug delivery systems work
ii) Examples of the drug delivery systems from the scientific literature (make sure that you reference primary research papers). You can refer to more than one example of each drug delivery system e.g. nanoparticles can be formulated in lots of different ways.
iii) Informative figures to help convey information (make sure figures are clearly annotated, and if adapted from a published paper make sure to include a reference)
iv) Discussion of the main advantages and disadvantages of each drug delivery system. e.g. ease of formulation, cost, stability, specificity, immunogenicity etc.
3. Conclusion
A conclusion should link back to the essay question, briefly restate your main points and highlight the significance of the topic.
4. Bibliography
References should be presented in the APA 7th style.
What criteria will be used to grade my essay?
Essays will be assessed on the following criteria for assignment help
Scientific content. (have you demonstrated a good understanding of complex scientific information?)
Correct interpretation of previously published data. (have you included reference to and discussion of relevant research papers? Have you interpreted the main findings of the research papers correctly?)
Structure and presentation. (have you structured your essay logically and have you used informative headings and subheadings?)
Use of informative figures. (do figures help to communicate key information, are they annotated properly?)
Use of appropriate language (scientific/medical terminology where appropriate).
(it is very important that you try to write using your own words. If your essay consists of large sections of text copy and pasted directly from other sources, it suggests a lack of understanding. Try to use scientific language and avoid colloquialism (language used for casual conversation)
Referencing. (Make sure that you use the APA 7th referencing style as instructed)
Solution
DEFINITION:
Without affecting non-targeted cells, tissues and organs "Drug delivery is a formula that provides therapeutic agents specifically to target cells.
The common used drug delivery system (DDS);
? Oral. (Mouth)
? Topical. (Skin)
? Trans-mucosal. (Nasal, Buccal, Sublingual, Vaginal, Rectal, Ocular)
? Parenteral. (Injection directly into the systemic circulation)
? Inhalation.
DDS is further divided into 2 main types;
1. Conventional Drug delivery system.
2. Novel Drug delivery system (Luo et al. 2020).
i. CONVENTIONALLY DRUG DELIVERY SYSTEM:
The conventional drug delivery method is the absorption of the drug through the cell membrane. This system includes;
? Oral Delivery
? Buccal/ Sublingual Delivery
? Rectal Delivery
? Intravenous Delivery
? Intramuscular Delivery
? Subcutaneous Delivery
Each of these delivery systems has some pros and cons.
ii. NOVEL DRUG DELIVERY SYSTEM (NDDS) :
Figure 1: Drug delivery system
(Source: Luo et al. 2020)
Figure 2: Overview of targeted drug delivery
(Source: Luo et al. 2020)
To help with this system, Medications are needed where the body distributes pharmaceutical agents to specific targets and achieves the desired response.
NDDS helps to improve the drug potency, A safe therapeutic effect is produced through the release of controlled drugs with greater protection and is directly targeted at the auction site.
Advantages of Novel in comparison with Conventional:
at the right time and at the right site It delivers the maximum dose. The amount of drugs given in the conventional method is allowed to be reduced in NDDS. The frequency of drug doses in NDDS is also reduced to create the desired effect in the workplace. Side effects are again reduced in NDDS because the desired amount of drug product is allowed to be delivered to the desired site (Sur et al. 2020).
More organic availability is required for this. It is released as soon as the drug is ingested in the conventional way and allows its effects to be created which cause fluctuations of the drug in the blood depending on the dosage form used. Therefore, NDDS is given priority to maintain the drug level in blood. Patients feel more comfortable due to better therapy and increased quality of life.
MODES OF NOVEL DRUG DELIVERY SYSTEM:
1. Drug Delivery System Targets
The concentration of drugs in this system increases over time in somebody parts compared to others. It is known as a smarter drug delivery system than others.
2. Controlled Drug Delivery System.
In this system, the correct dose of the drug is introduced into the body to provide long-term activity. This increases the effectiveness of the drug and reduces side effects.
3. Modulated drug delivery system:
This system involves various parameters that control the release of the drug, for example, physical, chemical, biochemical, physics or electrical.
Figure 3: Characteristics of drug delivery system
(Source: Sur et al. 2020)
CHARACTERISTICS DESIRABLE IN A DRUG DELIVERY SYSTEM:
The blood-brain obstruction, physiological or biological barriers drugs can easily penetrate and cross them. The target reaches the produces its effects according to the needs and requirements of the patient for a specified period. It should be ensured that the drug concentration should be within the (MEC) and (MTC). The bioavailability of drugs in the bloodstream is affected.
Transporting the drug directly to the site of action without affecting any other uninfected tissue. Provide a controlled supply of drugs. Drug transfer indirect drug activity without effect other connective tissue Transport High durability it can be balanced under various physics parameters. Easily managed, safe and reliable. It has to be cost-effective.
MAJOR CHALLENGES FOR DRUG DELIVERY SYSTEM:
Here is a definite challenge for the eye medicine delivery system. The maximum drug concentration at the auction site with greater therapeutic efficacy is to be achieved therapeutically. High-density solutions on the ocular surface can cause toxicity or damage to other cells. Poor bioavailability of ocular surface drugs can occur for a variety of reasons so vent solution extraction, tear distillation, lacrimation, conjunctival absorption, and more. For this,e reasons very small amounts are accepted to be Exploited unilaterally.
AREAS TO BE RESEARCHED FOR A BETTER DRUG DELIVERY SYSTEM:
Scientists are studying and use to identify always that how diseases are evolving and spreading further. They also use to study how our bodies are responding in response to those diseases and what is the effect of genetics and the environment on it (Rajpoot et al. 2020).
1. Crossing the Blood-Brain Barrier (BBB) in Brain Diseases and Disorders:
Different cells of the body use it to exchange basic substances like blood from the CNS. It helps to prevent the identification of elements that can enter the brain and damage cells. It is very normal and basic to administer drugs to the brain to get an effective response for a specific disease of the Brain (e.g. Parkinson's, Alzheimer's, brain tumours). Better strategies need to be developed to overcome this problem.
An ultrasound technique is performed that damages the brain in moments and safely passes the BBB so that the drug directly targets the tumour cells without using any treatment method.
2. Enhanced Drug Delivery to the Targeted Intracellular:
The immune system protects the organ against any harmful substances, so the cells detect and dispose of foreign particles or any other dangerous molecule. Drugs bound to carriers or vehicles to deliver targets are considered foreign particles for them. While scientists are working to develop authentic and useful treatment methods for delivering drugs to targets, more effective research is needed to ensure that drugs reach the site for their effects.
As a priority, in the carrier delivery systems are going to be improved to develop drugs in response to cellular defence, drug delivery to the intracellular target site, and molecular signalling.
3. Merging Diagnosis and Treatment
Drug delivery systems extend far beyond treatment. Someday with the use of imaging technology, physicians are going to identify and manage their patients in just one step. This new technique is known as theranostics (Rajpoot et al. 2020).
I. LIPOSOMAL AND TARGETED DRUG DELIVERY SYSTEM
A. Modification of liposomal drug targeting
Phospholipids are the result of polar shells between amphiphilic and follicle solutions in nature. An attractive drug delivery system analogous to cell membrane structurally and composition-wise as well is a liposome. liposomes are non-toxic,non-immunogenic, and biodegradable amphiphilic molecules. Biocompatibility, Biodegradability, Reduced toxicity, are included in liposomes, they have various important properties; motivate the pharmacokinetic profiles of the loaded drug.
Figure 4: Modification of liposomal drug targeting
(Source: Rajpoot et al. 2020)
Liposomes serve to deliver targeted drugs. Liposomes have been used as pharmaceutical transporters for decades, where liposomes can react selectively at the cellular and molecular levels with further growth and change.
HISTORY:
The invention of liposomes was introduced in the 1960. With the passing days different adoptions can be seen in phospholipid bilayer structures and it becomes a single bilayer structure and it is further termed as liposomes. Gregorian has established the process of encircling of liposomes which can be termed as a drug delivery system. Different kinds of contemporary processes have been established for the preparation of different beak and unilamellar liposomes. It is considered as the end in enhancing the efficiency and homogeneity of normal liposomes.
In the initial stage, the application of liposomes was seen as the form at low-pressure conditions, which was low yield. On the other hand, higher pressure systems were invented for higher yield. With the sonication and homogeneity technique, it was not possible to produce LUV with diameters of less than 50 nm. At the same time, the advancement of the microfluidic mixing procedure has enhanced the ability to scale in terms of the production of LUV within the range of 20–50 nm (Rajpoot et al. 2020).
From the 1st generation of liposomes, it is seen that the invention was quickly cleared by the reticuloendothelial system (RES) after completing the step of administration. Now, it can be said that the first strategy executed orderly to generate long-circulating liposomes by making an alteration of their attributes along with composition and size. At the same time, it is analysed that different types of small liposomes are not as clear as the RES. With the help of further improvement, it is possible to offer some formulations which are comparatively more stable and help in enhancing the deep ocean at the sight of liposomes. With the help of increased permeability and retention impacts, it is possible to increase the deposition and this is considered as one of the significant procedures.
The impacts of ERP can be observed as a procedure, which is possible with the help of a stronger extraversion of macromolecules that were first introduced from tumour blood vessels. After that, they are retained in a massive way in tumour tissues, infarcts and in some infected areas which are greater than the normal tissues. In addition to that after considering some individual studies, it is observed that 100 NM liposomes had been introduced as the most proper one for tumour delivery. On the other hand, the planning of arranging the liposomes can be seen in utilising wide phospholipids which consist of higher TM compared with unsaturated phospholipids and its flow time and retention is comparatively higher for the large vesicles.
In addition to that, the surface alteration of different repo sums has been applied to keeping away from the take up of RES. After considering the earlier examinations or investigations on sialic and ganglioside subsidiaries, for example, GM1 for the enhancement of the stability copies of different erythrocyte membrane surfaces. After completing the next steps of the investigation it can be said that the utilised hydrophilic polymers, for example, PEG17 is used for increasing the stable flow of different liposomes
It is also observed that after making and alteration of liposomes by the end goal, that is considered as a steady clean it has been introduced on it. It helps in the evolution of plasma protein binding and ensuing RES uptake. 20 types of surface alterations help in the enhancement of purported stealth liposomes. It is seen that these liposomes work more effectively because they are much capable in imitating the biomembranes. In this context, it can be said that Doxil is considered as one of the common stealth liposomes, which consists of PEG surface covering and that has exhibited a further development circulation time and safety profile in terms of utilisation in DOX chemotherapy.
At the end, the next generation of liquids some have influenced to implement direct atomic focusing which is operated by making a connection with site explicit ligands to the liposomal surface. Along with the methodology medication within the cells are enhanced by keeping a target site with the support of receptor-mediated endocytosis. The material which is collected that is endocytosed is then exposed in the form of acidic lysosomal compartmentalization and hydrolysis with the help of various chemicals. It is possible by bringing about diminished biological action. In this context, a problem can be identified for drugs that are especially delicate to this procedure of degradation, for example, peptidic and nucleic acid drugs.
These medications and procedures are trying to empower the process of entering the load into the cytosol are worthwhile and also assist to relieve this issue. Besides, the further improvement included the demonstration of a liposome, which consolidated long flow properties and marketability. Doxil was utilized as an anticancer drug and showed work on in vivo activity.
B. CLASSIFICATION OF LIPOSOMES:
After considering the structures of Liposomes, the classifications can be made in accordance with different types of composition and applications, structural parameters and several procedures, which include sonication, thin-film hydration, detergent dialysis, French press, reverse-phase evaporation, ethanol injection and extrusion (Khodashenas et al. 2020). Liposomes are proven according to the number of lipid bilayers in the colloidal structure, unilamellar liposomes consisting of multilamellar liposomes with one lipid bilayer and multiple lipid bilayers.
Figure 5: Classification based on composition and applications of liposomes
(Source: Khodashenas et al. 2020)
.png)
Figure 2 Classification based on structural parameters of liposomes
(Source: Khodashenas et al. 2020)
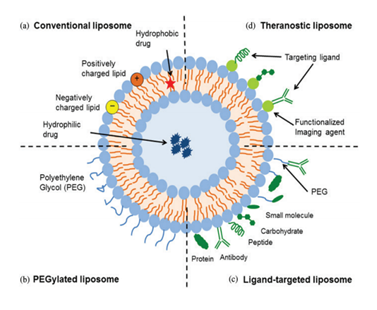
C. HOW DOES LIPOSOME TECHNIQUE WORKS:
Liposomes go into the stomach when swallowing the capsule. The acid in the stomach causes the capsule to quickly detach its subject, which is usually not a perfect setup for proper absorption. However, with the help of liposomal technology are formed in a structure called liposome which integrates bilayer molecules that help guide and transport the active ingredients so that they are efficiently absorbed. For this it works so uniquely (Jafari et al. 2021).
MECHANISM OF ACTION:
The liposome structure consists of a part of the aqueous solution in the hydrophobic membrane. Hydrophobic chemicals can easily be malted in a lipid membrane, for this reason, liposomes can carry both hydrophilic and hydrophobic.
For example, liposomes helped in cancerous cells treatment. Cells of Cancerous require a large number of fats for the growth and when they identify liposomes which are filled with anti-cancer drug as a source of their nutrition, cancerous cells target those liposomes and are absorbed by them. After all of its contents are released into the liposome site, the cancerous cells begin to die rapidly.
D. TYPES OF LIPOSOMAL DRUG DELIVERY PLATFORMS:
Four types of liposomal delivery systems are,
i. Conventional liposome.
ii. Sterically stabilized liposome.
iii. Ligand-targeted liposome.
iv. Theranostic liposome.
E. ADVANTAGES:
It is seen that Liposomal strategies enhance the effectiveness of the drug and the therapeutic index in the body. It further increases the durability of the drug through encapsulation. These are non-toxic, biodegradable, flexible, fully biodegradable and non-resistant to systemic and non-systemic administration (Alinejad Raiss & Hashemzadeh, 2020). The toxicity of encapsulated agents is reduced.
F. DISADVANTAGES:
Solubility is a bit less and it has a short half-life, for example, hydrolysis and oxidation type reactions. There may be leaks or infusions of encapsulated drugs can be seen. The production cost is comparatively higher, therefore, its retail price will also be higher. The cause of instability is fusion. Rapid changes of liposomes from the bloodstream by phagocytic cells of RES is a major barrier to liposome drug supply (Alipour, Alimohammady, Yumashev & Maseleno, 2020).
G. CHALLENGES IN LIPOSOMAL DRUG DELIVERY SYSTEM:
One of the challenges that will affect the development of a successful liposomal market is the possibility of cytotoxicity. In some cases, It has been found that liposomes have shown an effect quickly after their administration which is certainly not beneficial. It has detected which charged liposomes are ultimately toxic. Nevertheless, there is a possibility that some solvents such as ethanol and ether may be present in trace amounts during liposome production depending on the method (Raval et al. 2018).
Challenges related to production come down to drug infiltration, batch switching, effective disinfection methods, durability issues, and most common scalability issues. Multiple liposome production can also raise problems. To get over these problems liposomes are mixed with expensive raw materials so this strategy does not depend on effective costs.
CONCLUSION:
Liposomes are shaped cells of phospholipids, the basic components of people cell walls. The drug is bound to a liposome that binds it as a ‘payload’ which actually protects it from initial decay in the body. The main advantage of the liposome technique is its bioavailability, which aids in treatment by increasing the effect of the drug and reducing the dose. The liposome is used in cancer patients treatment. Since this strategy alters the pharmacokinetics of the drug (i.e. absorption, distribution, metabolism, and defecation), it can solve many problems by providing controlled drugs. Liposomes are classified according to composition and application and structure.
REFERENCE:
Alinejad, A., Raissi, H., & Hashemzadeh, H. (2020). Development and evaluation of a pH-responsive and water-soluble drug delivery system based on smart polymer coating of graphene nanosheets: an in silico study. RSC Advances, 10(52), 31106-31114.
Alipour, E., Alimohammady, F., Yumashev, A., & Maseleno, A. (2020). Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug. Journal of Molecular Modeling, 26(1), 1-8.
Jafari, Z., Baharfar, R., Rad, A. S., & Asghari, S. (2021). Potential of graphene oxide as a drug delivery system for Sumatriptan: a detailed density functional theory study. Journal of Biomolecular Structure and Dynamics, 39(5), 1611-1620.
Khodashenas, B., Ardjmand, M., Baei, M. S., Rad, A. S., & Akbarzadeh, A. (2020). Conjugation of pectin biopolymer with Au?nanoparticles as a drug delivery system: Experimental and DFT studies. Applied Organometallic Chemistry, 34(6), e5609.
Luo, Z., Liu, C., Quan, P., Yang, D., Zhao, H., Wan, X., & Fang, L. (2020). Mechanistic insights of the controlled release capacity of polar functional group in transdermal drug delivery system: the relationship of hydrogen bonding strength and controlled release capacity. Acta Pharmaceutica Sinica B, 10(5), 928-945.
Rajpoot, K., Tekade, M., Pandey, V., Nagaraja, S., Youngren-Ortiz, S. R., & Tekade, R. K. (2020). Self-microemulsifying drug-delivery system: ongoing challenges and future ahead. In Drug Delivery Systems (pp. 393-454). Academic Press.
Raval, J. P., Chejara, D. R., Ranch, K., & Joshi, P. (2018). Development of injectable in situ gelling systems of doxycycline hyclate for controlled drug delivery system. In Applications of Nanocomposite Materials in Drug Delivery (pp. 149-162). Woodhead Publishing.
Sur, S., Rathore, A., Dave, V., Reddy, K. R., Chouhan, R. S., & Sadhu, V. (2019). Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Structures & Nano-Objects, 20, 100397.

.png)


.png)
~5.png)
.png)
~1.png)





















































.png)






